-
摘要:
太赫兹波所具有的无损性以及大量生物分子在太赫兹频段的指纹特性,使其在医学成像领域有着良好的应用前景。本文首先简要概述了太赫兹的医学成像技术手段,其次分别介绍了太赫兹在离体、活体组织中成像的研究现状。生物组织中的水会对太赫兹波产生强吸收,使得成像对比度受限。目前,为了减少组织中的水对成像的影响,针对离体组织的太赫兹成像大多需要进行切片、脱水等预处理,活体中的成像则主要应用在浅表组织。文章重点介绍了活体成像中有望提高太赫兹成像对比度的纳米粒子造影剂,最后对太赫兹医学成像的发展进行了展望。
Abstract:Terahertz wave has non-destructive nature and fingerprint characteristics for a large number of biomolecules, thus has a good application prospect in the field of medical imaging. In this review, we presented a brief introduction on the terahertz medical imaging systems, and the applications of terahertz medical imaging in biological tissues from in vitro to in vivo. Terahertz wave can be strongly absorbed by water, then the terahertz imaging contrast will be severely deteriorated in vivo. So the terahertz medical imaging was mainly used for detecting epidermal tissues or biological tissues with pretreatments, including excision, dehydration and so on. This review also concluded the recent development of nanoparticle contrast agents for improving the contrast of terahertz imaging in vivo. Finally, the future development of terahertz medical imaging was predicted.
-
Key words:
- terahertz /
- medical imaging /
- contrast /
- contrast agents
-

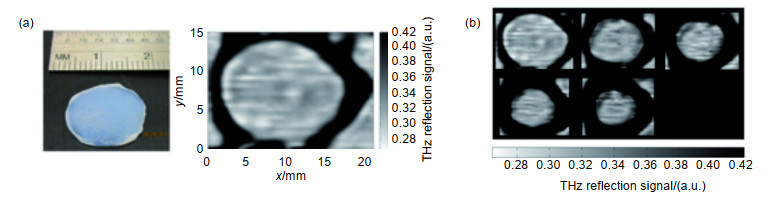
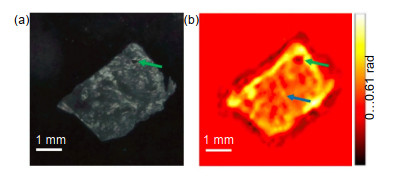
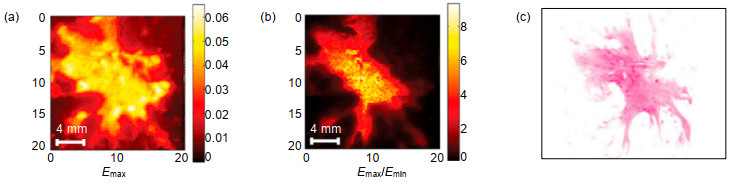
Overview: Terahertz, ranging from 0.1 THz to 10 THz, is situated in the frequency regime between optical and electronic techniques. Recently, with the rapid development of terahertz technology, it is widely applied in several fields such as material science, physics, chemistry, biology, and medicine. Due to the unique characteristics including low photon energy, excellent penetration ability through non-conducting materials and distinctive molecular fingerprints identification, terahertz medical imaging has become a promising imaging modality to date. It has been a significantly complementary medical imaging method, compared to other methods like magnetic resonance imaging (MRI), computed X-ray tomography (CT) and positron emission tomography (PET). And there has been an increasing interest in terahertz imaging for medical applications within the last few years, meanwhile, more and more terahertz imaging studies are being reported. In this review, we present a brief introduction on the terahertz imaging systems, and the applications of terahertz medical imaging from in vitro to in vivo. The essential mechanisms of terahertz medical imaging are based on the differences in water content and structural variations of tissues. But the abundant water in living tissues will strongly absorb terahertz wave, and lead to severely deteriorated imaging contrast. As a result, the terahertz medical imaging is mainly used in vitro or epidermal tissues. In most cases, the in vitro tissues should be pretreated with the processes including frozen sections, paraffin sections and so on. Many tissues have been studied by terahertz medical imaging in both human and animal models. Particularly, cancerous tissues of digestive system, reproductive system, integumentary system and respiratory system are focused. Brain, liver, breast tumors, for example, have been studied after different pretreatments. Fresh tissues directly excised from these tumors are also utilized to assess both water content and structural variations. While applied in vivo, skins are the main detected projects due to the penetration limit caused by water. In addition, some other methods have also been proposed to promote the application of terahertz medical imaging in the living body, such as endoscopy and penetration enhancing agents. Particularly, the nanoparticles contrast agents for terahertz medical imaging have been developed recently. This review concluded investigation of these contrast agents, including gold nanorods, gadolinium oxide nanoparticles, and superparamagnetic iron oxide nanoparticles. It seems that these contrast agents could enhance the imaging contrast largely, and would promote the application of terahertz medical imaging in vivo. Finally, the future development of terahertz medical imaging is prospected.
-

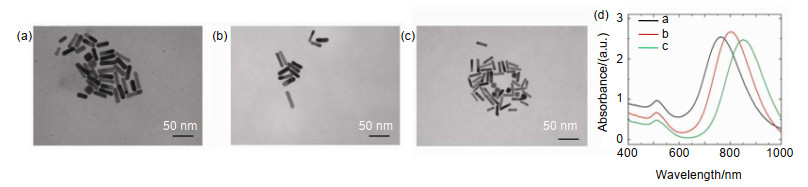
-
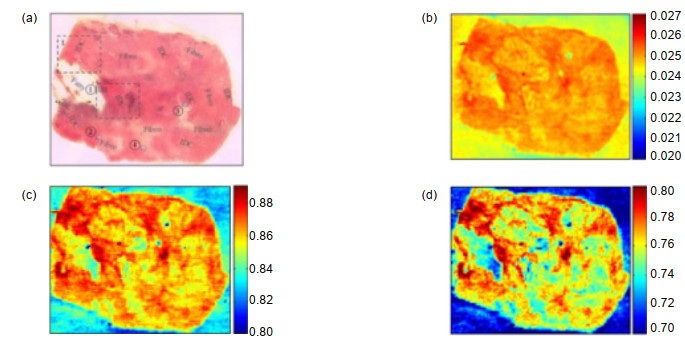
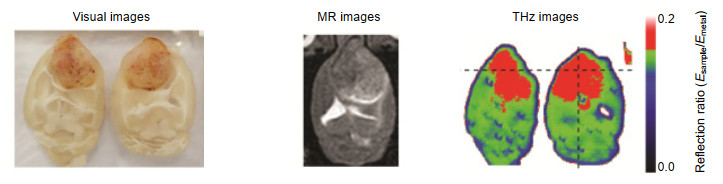
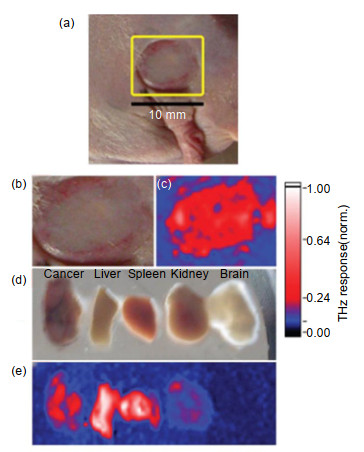
图 13 体内和体外肿瘤的太赫兹图像。(a),(b)体内肿瘤的光学图像;(c)体内肿瘤的太赫兹图像;(d)肿瘤、肝脏、脾脏、肾脏和大脑切片的光学图像;(e)切片的太赫兹图像[36]
Figure 13. In vivo and ex vivo terahertz molecular images of tumors. (a), (b) Visible images of the mouse with an A431 tumor in vitro; (c) Terahertz image of (b); (d) Visible images of the tumor, liver, spleen, kidney, and brain samples; (e) Terahertz image of (d)[36]
表 1 太赫兹医学成像的应用
Table 1. Applications of terahertz medical imaging
-
[1] Yu C, Fan S T, Sun Y W, et al. The potential of terahertz imaging for cancer diagnosis: a review of investigations to date[J]. Quant Imaging Med Surg, 2012, 2(1): 33-45. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3496499
[2] Fan S T, He Y Z, Ung B S, et al. The growth of biomedical terahertz research[J]. Journal of Physics D: Applied Physics, 2014, 47(37): 374009. doi: 10.1088/0022-3727/47/37/374009
[3] Kawase K, Ogawa Y, Watanabe Y, et al. Non-destructive terahertz imaging of illicit drugs using spectral fingerprints[J]. Optics Express, 2003, 11(20): 2549-2554. doi: 10.1364/OE.11.002549
[4] Bowman T C, El-Shenawee M, Campbell L K. Terahertz imaging of excised breast tumor tissue on paraffin sections[J]. IEEE Transactions on Antennas and Propagation, 2015, 63(5): 2088-2097. doi: 10.1109/TAP.2015.2406893
[5] Rong L, Latychevskaia T, Chen C H, et al. Terahertz in-line digital holography of human hepatocellular carcinoma tissue[J]. Scientific Reports, 2015, 5: 8445. doi: 10.1038/srep08445
[6] Bowman T, El-Shenawee M, Campbell L K. Terahertz transmission vs reflection imaging and model-based characterization for excised breast carcinomas[J]. Biomedical Optics Express, 2016, 7(9): 3756-3783. doi: 10.1364/BOE.7.003756
[7] Wahaia F, Kasalynas I, Venckevicius R, et al. Terahertz absorption and reflection imaging of carcinoma-affected colon tissues embedded in paraffin[J]. Journal of Molecular Structure, 2016, 1107: 214-219. doi: 10.1016/j.molstruc.2015.11.048
[8] Ji Y B, Lee E S, Kim S H, et al. A miniaturized fiber-coupled terahertz endoscope system[J]. Optics Express, 2009, 17(19): 17082-17087. doi: 10.1364/OE.17.017082
[9] Oh S J, Kim S H, Jeong K, et al. Measurement depth enhancement in terahertz imaging of biological tissues[J]. Optics Express, 2013, 21(18): 21299-21305. doi: 10.1364/OE.21.021299
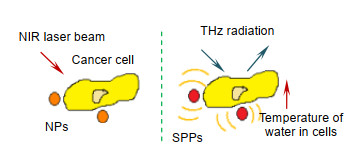
[10] Oh S J, Kang J, Maeng I, et al. Nanoparticle-enabled terahertz imaging for cancer diagnosis[J]. Optics Express, 2009, 17(5): 3469-3475. doi: 10.1364/OE.17.003469
[11] Lee D K, Kim H, Kim T, et al. Characteristics of gadolinium oxide nanoparticles as contrast agents for terahertz imaging[J]. Journal of Infrared, Millimeter, and Terahertz Waves, 2011, 32(4): 506-512. doi: 10.1007/s10762-011-9776-7
[12] Zhang R, Zhang L L, Wu T, et al. Contrast-enhanced continuous-terahertz-wave imaging based on superparamagnetic iron oxide nanoparticles for biomedical applications[J]. Optics Express, 2016, 24(8): 7915-7921. doi: 10.1364/OE.24.007915
[13] Huang Q Q, Zou Y, Zhong S C, et al. Silica-coated gold nanorods with high photothermal efficiency and biocompatibility as a contrast agent for in vitro terahertz imaging[J]. Journal of Biomedical Nanotechnology, 2019, 15(5): 910-920. doi: 10.1166/jbn.2019.2738
[14] Hu B B, Nuss M C. Imaging with terahertz waves[J]. Optics Letters, 1995, 20(16): 1716-1718. doi: 10.1364/OL.20.001716
[15] Wu L M, Xu D G, Wang Y Y, et al. Study of in vivo brain glioma in a mouse model using continuous-wave terahertz reflection imaging[J]. Biomedical Optics Express, 2019, 10(8): 3953-3962. doi: 10.1364/BOE.10.003953
[16] Mahon R J, Murphy J A, Lanigan W. Digital holography at millimetre wavelengths[J]. Optics Communications, 2006, 260(2): 469-473. doi: 10.1016/j.optcom.2005.11.024
[17] Mitchell H H, Hamilton T S, Steggerda F R, et al. The chemical composition of the adult human body and its bearing on the biochemistry of growth[J]. Journal of Biological Chemistry, 1945, 158(3): 625-637. http://cn.bing.com/academic/profile?id=ca880d406403979438c8266493cbc052&encoded=0&v=paper_preview&mkt=zh-cn
[18] Crawley D A, Longbottom C, Wallace V P, et al. Three-dimensional terahertz pulse imaging of dental tissue[J]. Journal of Biomedical Optics, 2003, 8(2): 303-307. doi: 10.1117/1.1559059
[19] Bennett D B, Taylor Z D, Tewari P, et al. Terahertz sensing in corneal tissues[J]. Journal of Biomedical Optics, 2011, 16(5): 057003. doi: 10.1117/1.3575168
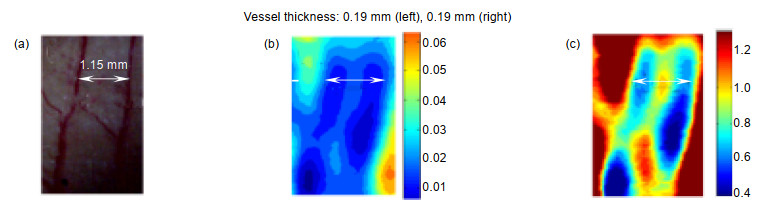
[20] Tseng T F, Yang S C, Shih Y T, et al. Near-field sub-THz transmission-type image system for vessel imaging in-vivo[J]. Optics Express, 2015, 23(19): 25058-25071. doi: 10.1364/OE.23.025058
[21] Fan S T, Ung B S Y, Parrott E P J, et al. In vivo terahertz reflection imaging of human scars during and after the healing process[J]. Journal of Biophotonics, 2017, 10(9): 1143-1151. doi: 10.1002/jbio.201600171
[22] Cassar Q, Al-Ibadi A, Mavarani L, et al. Pilot study of freshly excised breast tissue response in the 300 - 600 GHz range[J]. Biomedical Optics Express, 2018, 9(7): 2930-2942. doi: 10.1364/BOE.9.002930
[23] Kolesnikov A S, Kolesnikova E A, Popov A P, et al. In vitro terahertz monitoring of muscle tissue dehydration under the action of hyperosmotic agents[J]. Quantum Electronics, 2014, 44(7): 633-640. doi: 10.1070/QE2014v044n07ABEH015493
[24] Stylianou A, Talias M A. Nanotechnology-supported THz medical imaging[J]. F1000Research, 2013, 2(1): 100. http://d.old.wanfangdata.com.cn/OAPaper/oai_pubmedcentral.nih.gov_3869482
[25] Lee K, Jeoung K, Kim S H, et al. Measuring water contents in animal organ tissues using terahertz spectroscopic imaging[J]. Biomedical Optics Express, 2018, 9(4): 1582-1589. doi: 10.1364/BOE.9.001582
[26] Wallace V P, Fitzgerald A J, Shankar S, et al. Terahertz pulsed imaging of basal cell carcinoma ex vivo and in vivo[J]. British Journal of Dermatology, 2004, 151(2): 424-432. doi: 10.1111/j.1365-2133.2004.06129.x
[27] Fitzgerald A J, Wallace V P, Jimenez-Linan M, et al. Terahertz pulsed imaging of human breast tumors[J]. Radiology, 2006, 239(2): 533-540. doi: 10.1148/radiol.2392041315
[28] Ashworth P C, Pickwell-MacPherson E, Provenzano E, et al. Terahertz pulsed spectroscopy of freshly excised human breast cancer[J]. Optics Express, 2009, 17(15): 12444-12454. doi: 10.1364/OE.17.012444
[29] Oh S J, Kim S H, Ji Y B, et al. Study of freshly excised brain tissues using terahertz imaging[J]. Biomedical Optics Express, 2014, 5(8): 2837-2842. doi: 10.1364/BOE.5.002837
[30] Ji Y B, Oh S J, Kang S G, et al. Terahertz reflectometry imaging for low and high grade gliomas[J]. Scientific Reports, 2016, 6: 36040. doi: 10.1038/srep36040
[31] Tewari P, Bajwa N, Singh R S, et al. In vivo terahertz imaging of rat skin burns[J]. Journal of Biomedical Optics, 2012, 17(4): 040503. doi: 10.1117/1.JBO.17.4.040503
[32] Arbab M H, Winebrenner D P, Dickey T C, et al. Terahertz spectroscopy for the assessment of burn injuries in vivo[J]. Journal of Biomedical Optics, 2013, 18(7): 077004. doi: 10.1117/1.JBO.18.7.077004
[33] Sim Y C, Park J Y, Ahn K M, et al. Terahertz imaging of excised oral cancer at frozen temperature[J]. Biomedical Optics Express, 2013, 4(8): 1413-1421. doi: 10.1364/BOE.4.001413
[34] Oh S J, Maeng I, Shin H J, et al. Nanoparticle contrast agents for Terahertz medical imaging[C]//Proceedings of the 2008 33rd International Conference on Infrared, Millimeter and Terahertz Waves, 2008: 1-2.
https://ieeexplore.ieee.org/document/4665813/ [35] Oh S J, Choi J, Maeng I, et al. High-sensitivity terahertz imaging technique using nanoparticle probes for medical applications[C]//Proceedings of 2010 IEEE Photonics Society Winter Topicals Meeting Series, 2010: 52-53.
https://ieeexplore.ieee.org/document/5421967/?denied= [36] Oh S J, Choi J, Maeng I, et al. Molecular imaging with terahertz waves[J]. Optics Express, 2011, 19(5): 4009-4016. doi: 10.1364/OE.19.004009
[37] Oh S J, Huh Y M, Suh J S, et al. Cancer diagnosis by terahertz molecular imaging technique[J]. Journal of Infrared, Millimeter, and Terahertz Waves, 2012, 33(1): 74-81. doi: 10.1007/s10762-011-9847-9
[38] Cristian C R, Thomas S, Vasile D, et al. Research on functionalized gadolinium oxide nanoparticles for MRI and THz imaging[C]//Proceedings of 2018 International Conference and Exposition on Electrical And Power Engineering (EPE), 2018: 646-649.
https://www.researchgate.net/publication/329616698_Research_on_Functionalized_Gadolinium_Oxide_Nanoparticles_for_MRI_and_THz_Imaging [39] Park J Y, Choi H J, Nam G E, et al. In vivo dual-modality terahertz/magnetic resonance imaging using superparamagnetic iron oxide nanoparticles as a dual contrast agent[J]. IEEE Transactions on Terahertz Science and Technology, 2012, 2(1): 93-98. doi: 10.1109/TTHZ.2011.2177174
[40] Bowman T, Walter A, Shenderova O, et al. A phantom study of terahertz spectroscopy and imaging of micro- and nano-diamonds and nano-onions as contrast agents for breast cancer[J]. Biomedical Physics & Engineering Express, 2017, 3(5): 055001. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=IOP_9363252
[41] El-Shenawee M, Vohra N, Bowman T, et al. Cancer detection in excised breast tumors using terahertz imaging and spectroscopy[J]. Biomedical Spectroscopy and Imaging, 2019, 8(1-2): 1-9. doi: 10.3233/BSI-190187
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: