Review of the development of optical coherence tomography imaging navigation technology in ophthalmic surgery
-
摘要
在眼科显微手术中,传统的术中成像方式由于缺少深度信息,限制了内部结构和手术器械的可视化。光学相干层析成像技术(OCT)是一种非接触式断层成像技术,由于其能提供深度信息、非侵入、成像快、分辨率高等优点,被广泛应用于眼科手术的术中导航。典型的OCT设备可分为手持OCT和显微镜集成OCT。本文简要介绍了时域OCT和频域OCT的原理和发展,回顾了OCT眼科手术导航设备的发展历程,并对各个类别中有代表性的OCT系统进行了介绍,对其成像原理、性能、优缺点等进行了描述和对比,最后对该技术在眼科手术中的应用做出了总结和展望。
Abstract
During ophthalmic microsurgery, the visualization of internal structures is limited by traditional intraoperative imaging methods due to their lack of depth information. Optical coherence tomography (OCT) is a non-contact tomographic imaging technique that is widely used for intraoperative navigation in ophthalmic surgery because of its ability to provide depth information, non-invasiveness, fast imaging, and high resolution. Typical OCT devices can be divided into handheld OCT and microscope-integrated OCT. This article briefly introduces the mechanism and development of time domain OCT and fourier domain OCT, reviews the development of OCT ophthalmic surgical navigation devices, introduces representative OCT systems in each category, describes and compares their imaging principles, performance, advantages, and disadvantages, and finally concludes with a summary and outlook on the applications of this technology in ophthalmic surgery.
-
Overview
Overview: With the development of microsurgery, minimally invasive ophthalmic surgery has become the primary means for the treatment of eye diseases. Ophthalmic surgeries need to observe the structure under the surface and accurately locate the surgical instruments in real time. Conventional surgical operating microscope is difficult to use for ophthalmic intraoperative imaging due to its lack of depth information. Optical coherence tomography (OCT) is a non-contact tomography technology that can provide depth information during ophthalmic surgeries. It has been widely used in clinical ophthalmic surgery because of its non-invasive imaging mode, fast imaging speed, and high imaging quality.
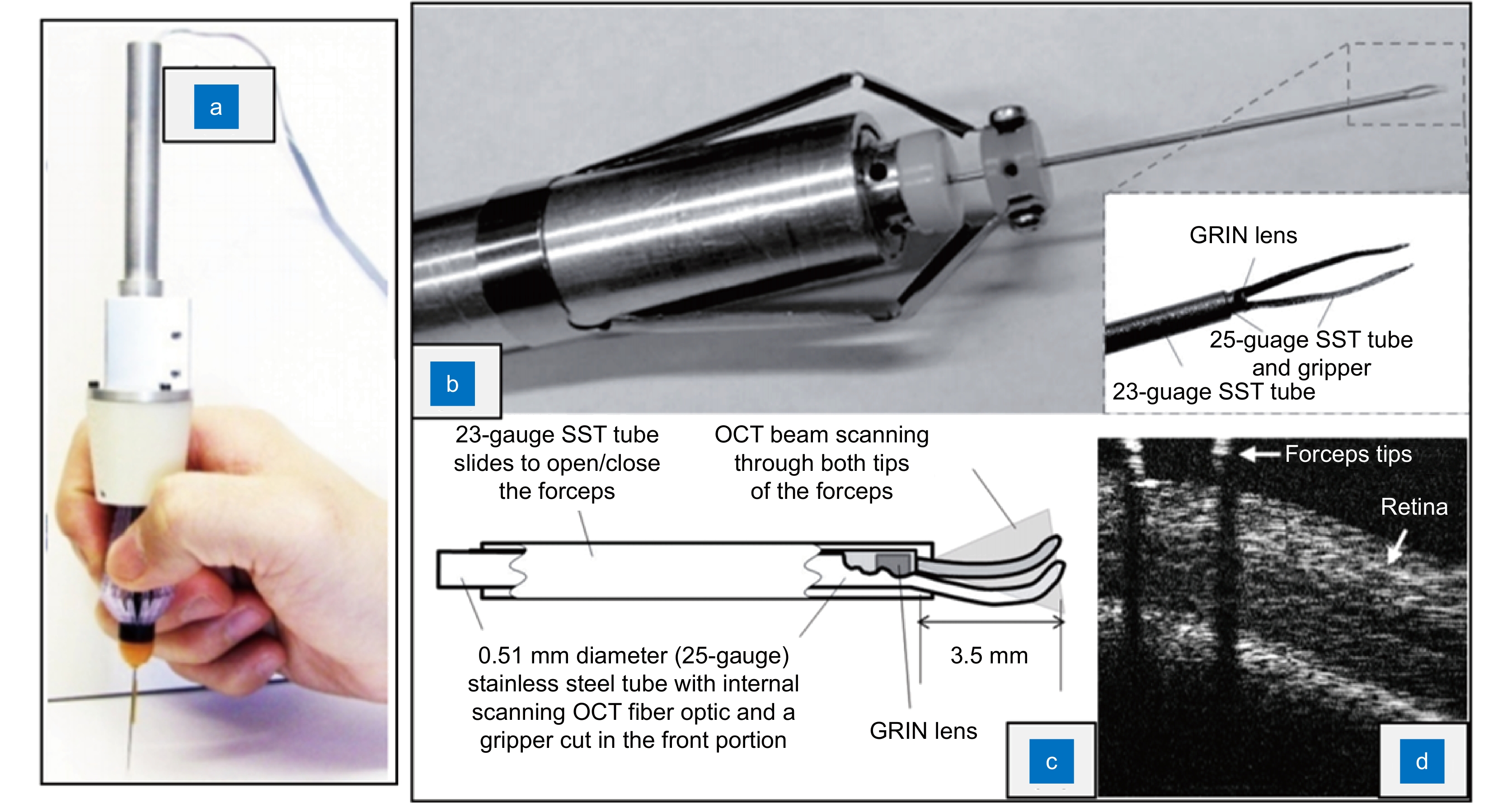
Typical intraoperative OCT devices can be divided into handheld OCT (HHOCT) and microscope-integrated OCT (MIOCT). Handheld OCT can be further divided into external HHOCT probe, needle-based HHOCT probe, and OCT-integrated surgical instrument. HHOCT can optimize the volume interference caused by traditional tabletop equipments. The external HHOCT probe has the advantages of non-contact and non-invasiveness. The needle-based HHOCT probe can enter the eye under minimally invasive conditions to image the fundus structure, while the OCT integrated surgical instrument can ensure the alignment between the image and the end of the instrument, which is conducive to the judgment of the position of the instrument during eye surgeries.
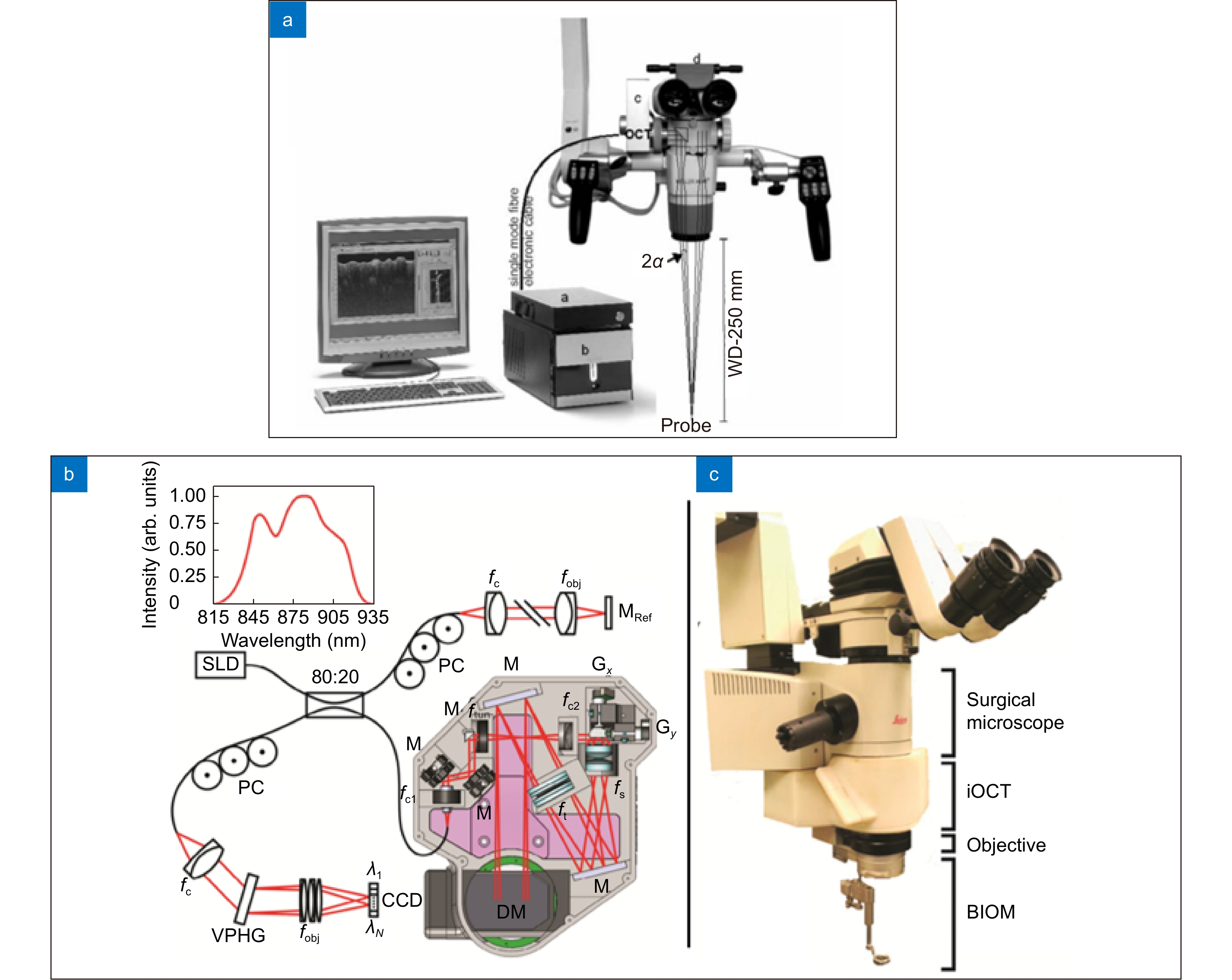
Microscope-integrated OCT is another way of intraoperative OCT imaging that is realized by integrating the optical system of both microscope and OCT. In this way, there is no need to interrupt the operation or add operators. At present, MIOCT real-time two-dimensional imaging is relatively mature and has been widely used in ophthalmic ssurgeries. With the development of graphics processing unit (GPU) and the introduction of swept frequency OCT (SS-OCT), intraoperative real-time three-dimensional imaging has become the future trend of MIOCT. However, there are still some problems in 3D OCT imaging, such as blurred structure surface, poor edge definition, and difficult recognition of surgical instruments. Improving image contrast is the key to solve the problems above. An effective approach is to use volume enhancement rendering algorithm for feature enhancement and shadow coloring. Another method is to use coloration in the process of volume rendering based on depth and intensity signals, and thus enhances the ability to recognize the retinal deformation and the contact between instrument and membrane.
The significance of OCT imaging in ophthalmic surgery has been proved in experiments on animal eyes, human eye models, and clinical cases. In recent years, commercial OCT surgical navigation equipment has already been widely used in ophthalmic clinical surgery. With the progress of image processing technology, image and ophthalmology, OCT surgical navigation equipment will further promote the innovation of ophthalmic surgery, and thus promote the development of the ophthalmology field.
-

-
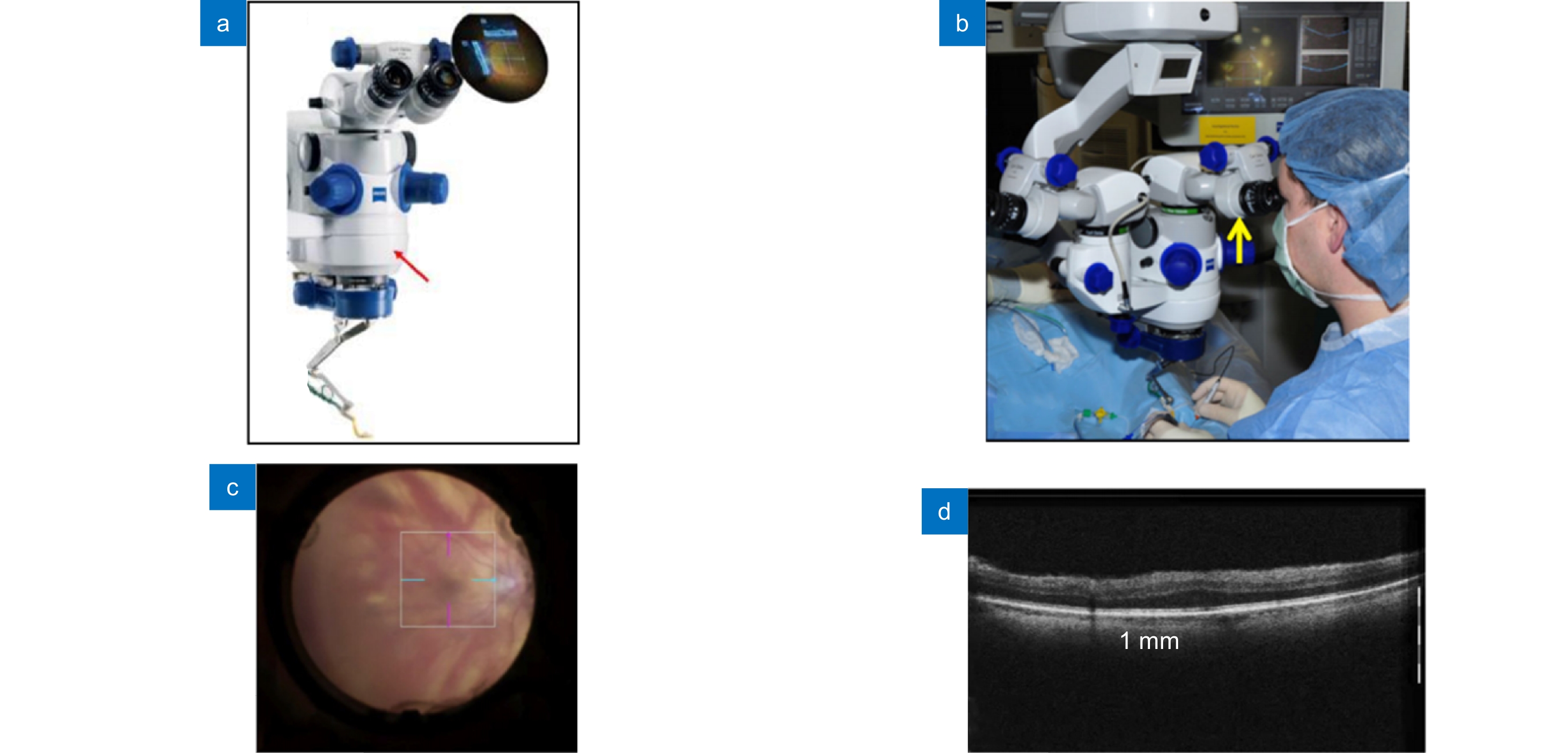
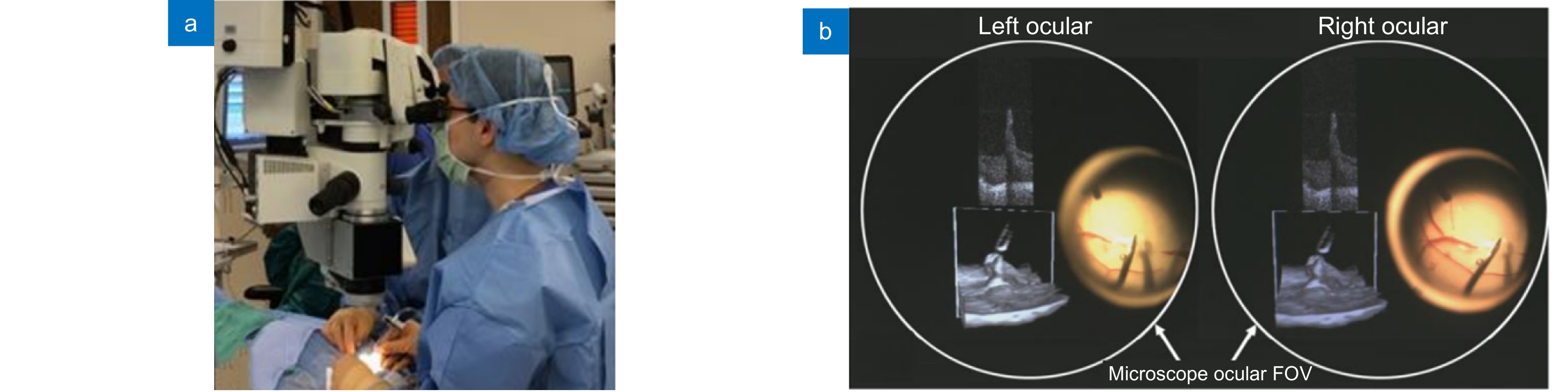
图 8 商业化MIOCT:蔡司RESCAN 700。(a) RESCAN 700机体[3];(b) 医生在手术中使用RESCAN 700[69];(c) RESCAN 700光学显微镜眼底成像[68];(d) RESCAN 700 OCT系统眼底成像[71]
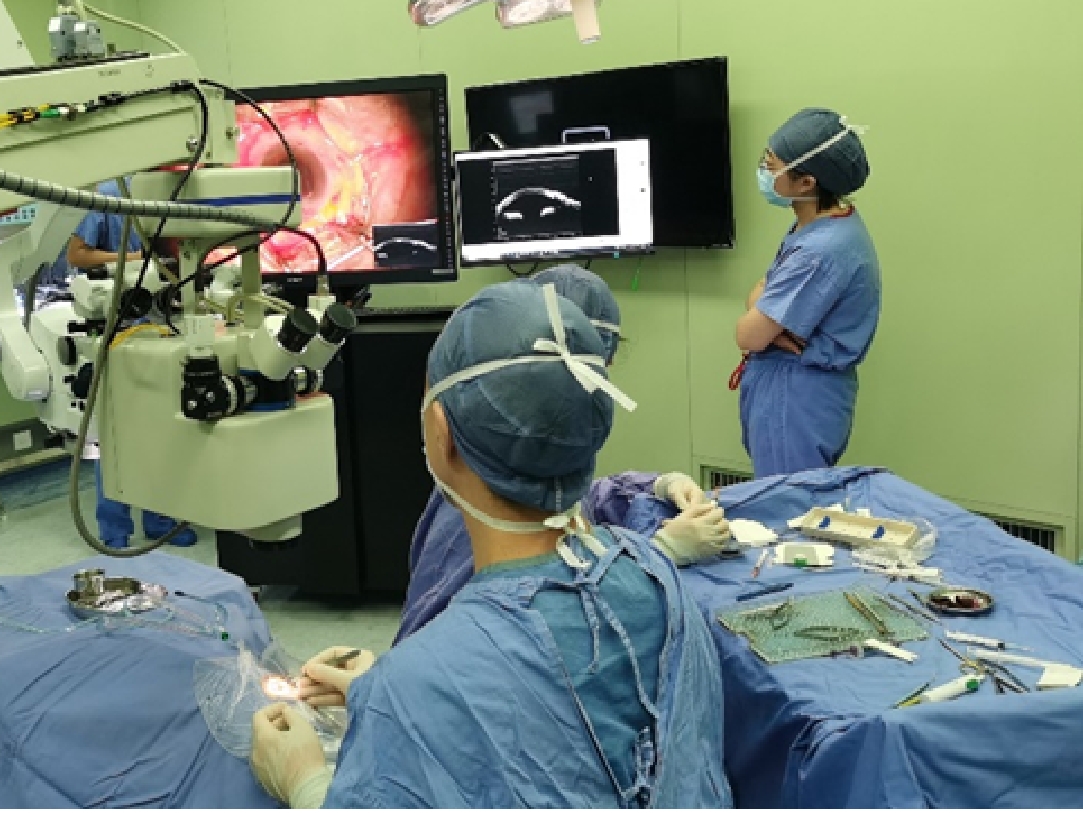
Figure 8. Commercialized MIOCT system: Zeiss RESCAN 700. (a) RESCAN 700 system[3]; (b) Surgeon using RESCAN 700 during ocular surgeries[69]; (c) Microscope imaging of the RESCAN 700 system[68]; (d) OCT imaging of the RESCAN 700 system[71]
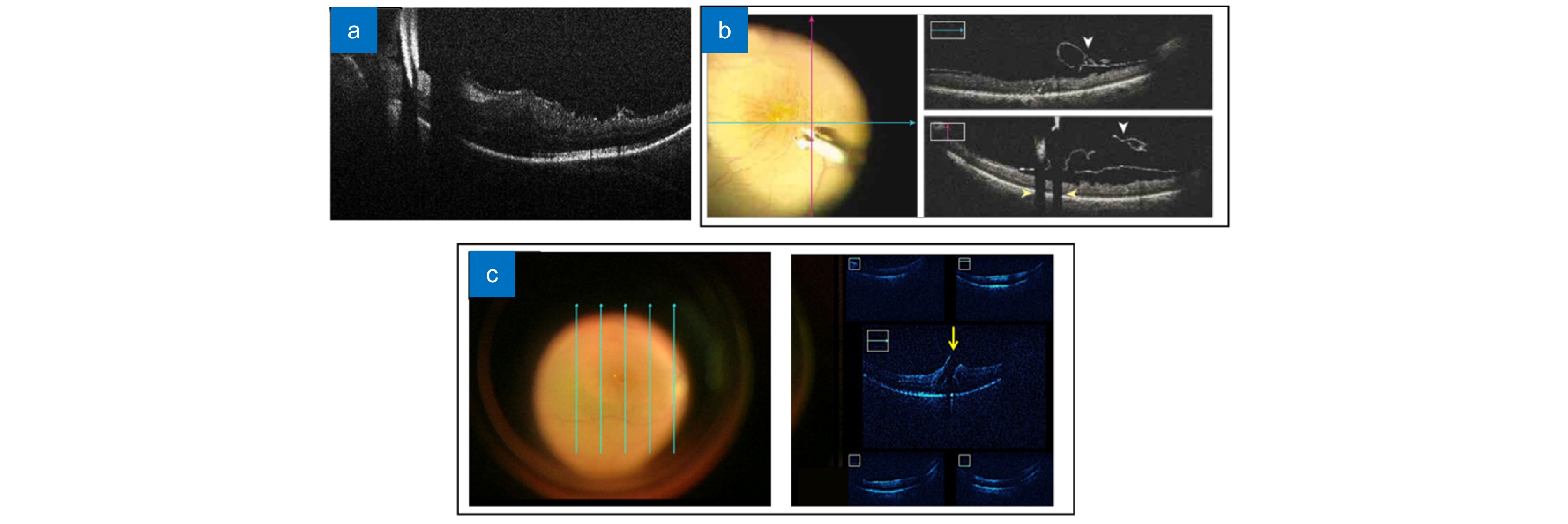
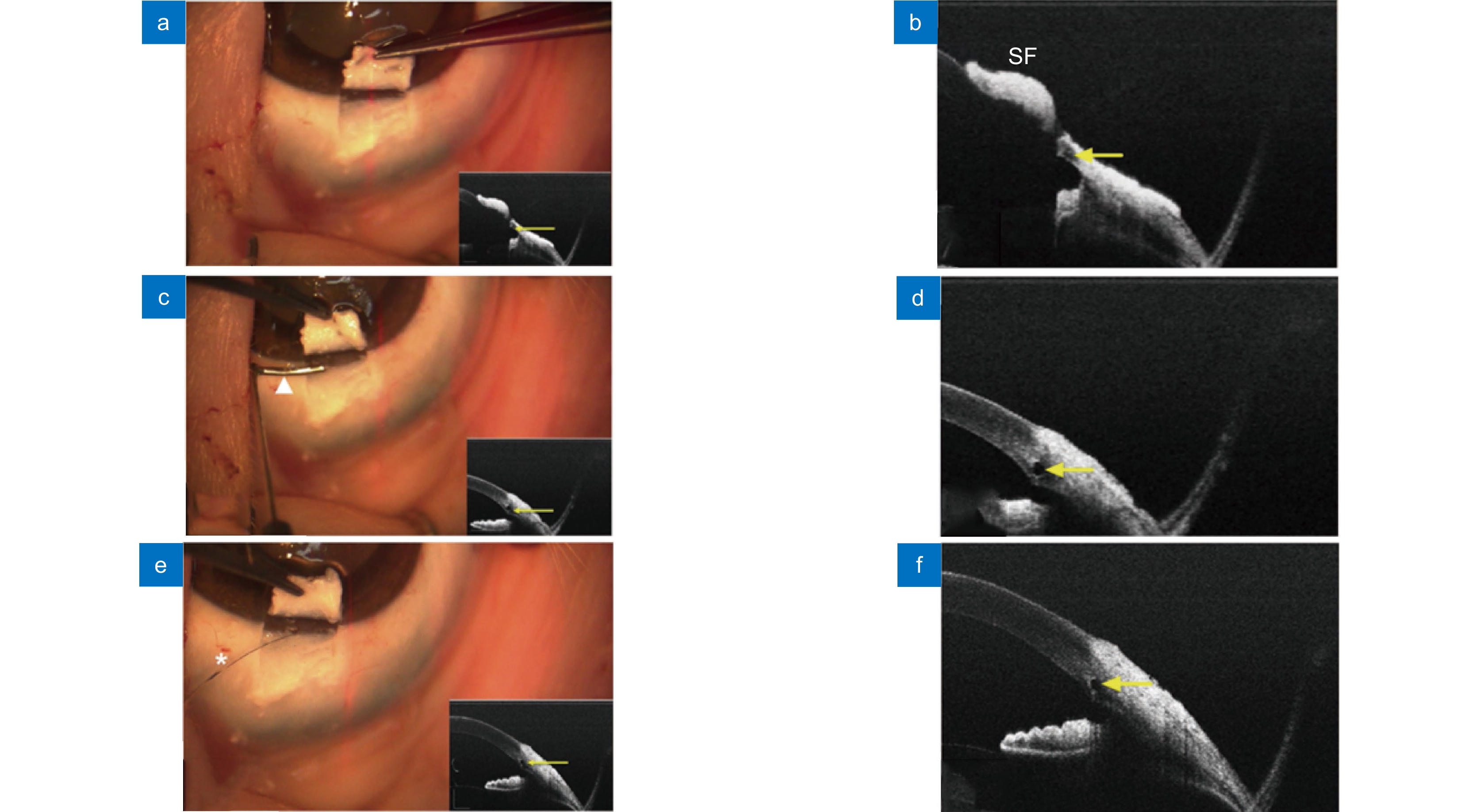
图 10 使用基于扫频OCT的MIOCT对眼前节手术(泪道成形术)成像[78]。(a, b) 切开浅层巩膜瓣后的MIOCT图像;(c, d) 插入小梁切刀后的MIOCT图像;(e, f) 借助MIOCT图像确认集束管扩张
Figure 10. Real-time images of anterior segment surgery(canaloplasty) from the microscope-integrated swept-source optical coherence tomography (MIOCT) system[78]. (a, b) MIOCT image after incision of a superficial scleral flap; (c, d) MIOCT image after insertion of a custom-made trabeculotomy; (e, f) Confirming expansion of the collector vessel using MIOCT image
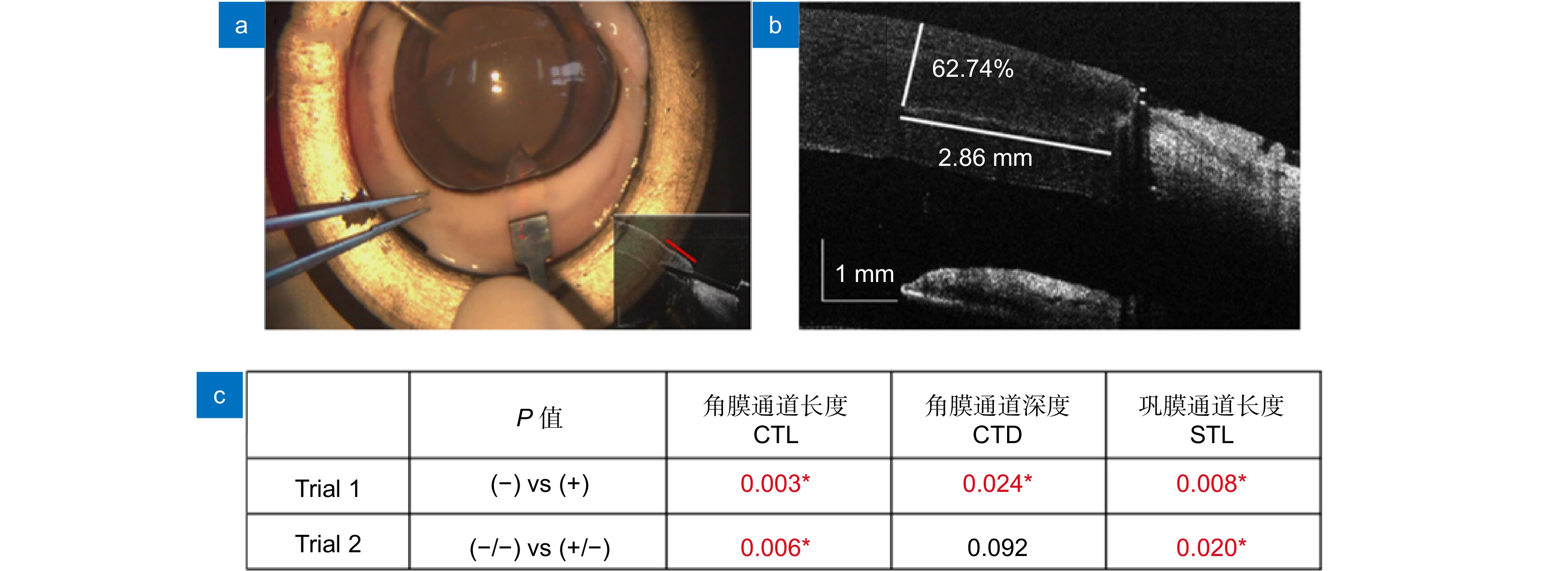
图 11 使用基于扫频OCT的MIOCT导航眼科手术操作的实验结果[77]。(a) 术中OCT成像;(b) 术后OCT切口分析;(c) 精度测试结果。Trial 1:使用(+)和未使用(-)MIOCT的对比;Trial 2:经MIOCT训练(+/-)和未经MIOCT训练(-/-)后使用传统显微镜的手术精度对比(*代表统计学显著不同)
Figure 11. Experimental results of ophthalmic surgeries navigated by swept-frequency OCT-based MIOCT. (a) Intraoperative OCT image; (b) Postoperative suture analysis using OCT; (c) Accuracy test results. Trial 1: comparison of the results with (+) and without (-) MIOCT; Trial 2: accuracy of traditional microscope guided surgeries with (+/-) and without (-/-) MIOCT training (* represents statistically significant difference)
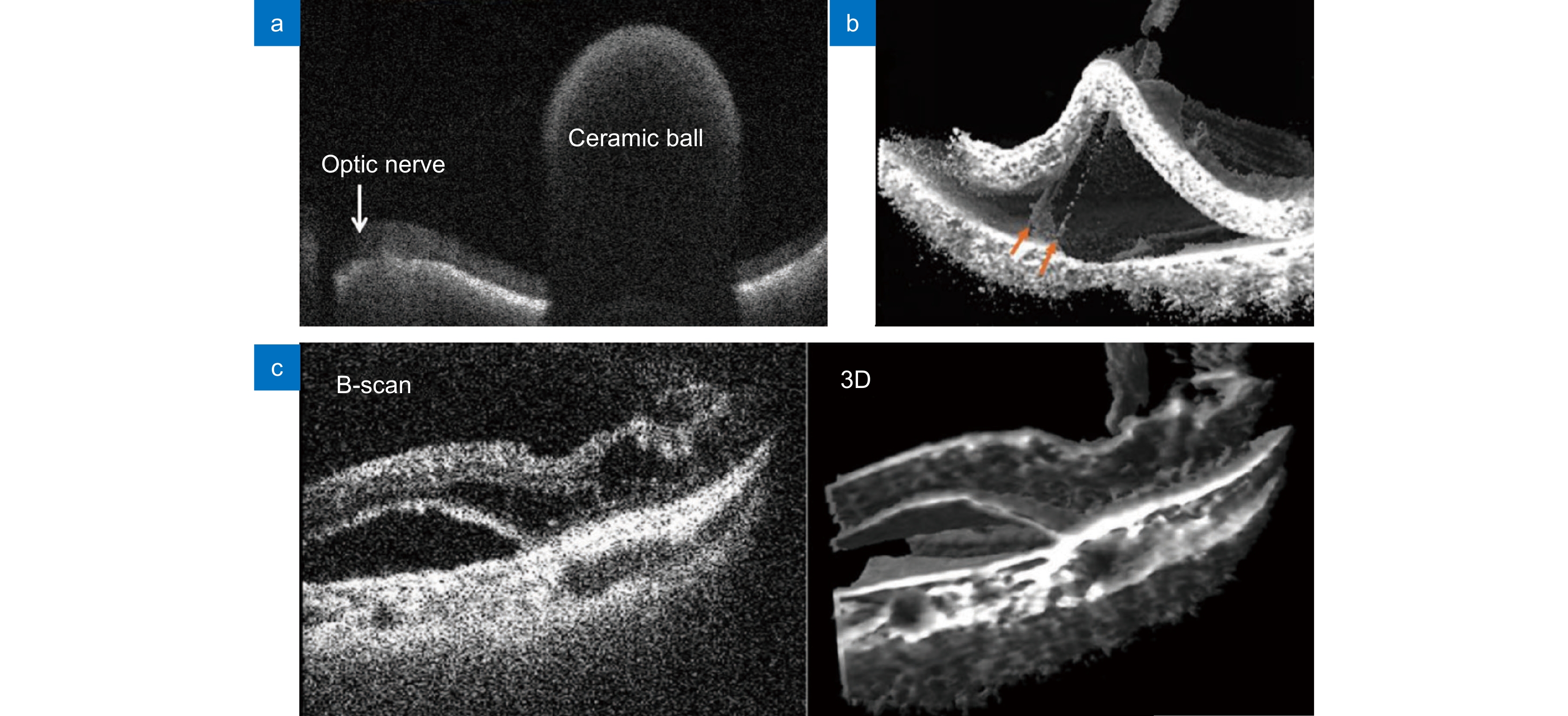
图 12 4D MIOCT实时成像。(a) 观测视网膜上的陶瓷球体[82];(b) 手术工具抓取视网膜色素上皮细胞层的实时图像[83];(c) 玻璃体切除术中视网膜下积液的二维和三维图像[84]
Figure 12. 4D MIOCT real-time imaging. (a) Observing a ceramic ball on the retina[82]; (b) Real-time image of a surgical tool grasping the retinal pigment epithelial cell layer[83]; (c) 2D and 3D images of subretinal fluid during vitrectomy[84]
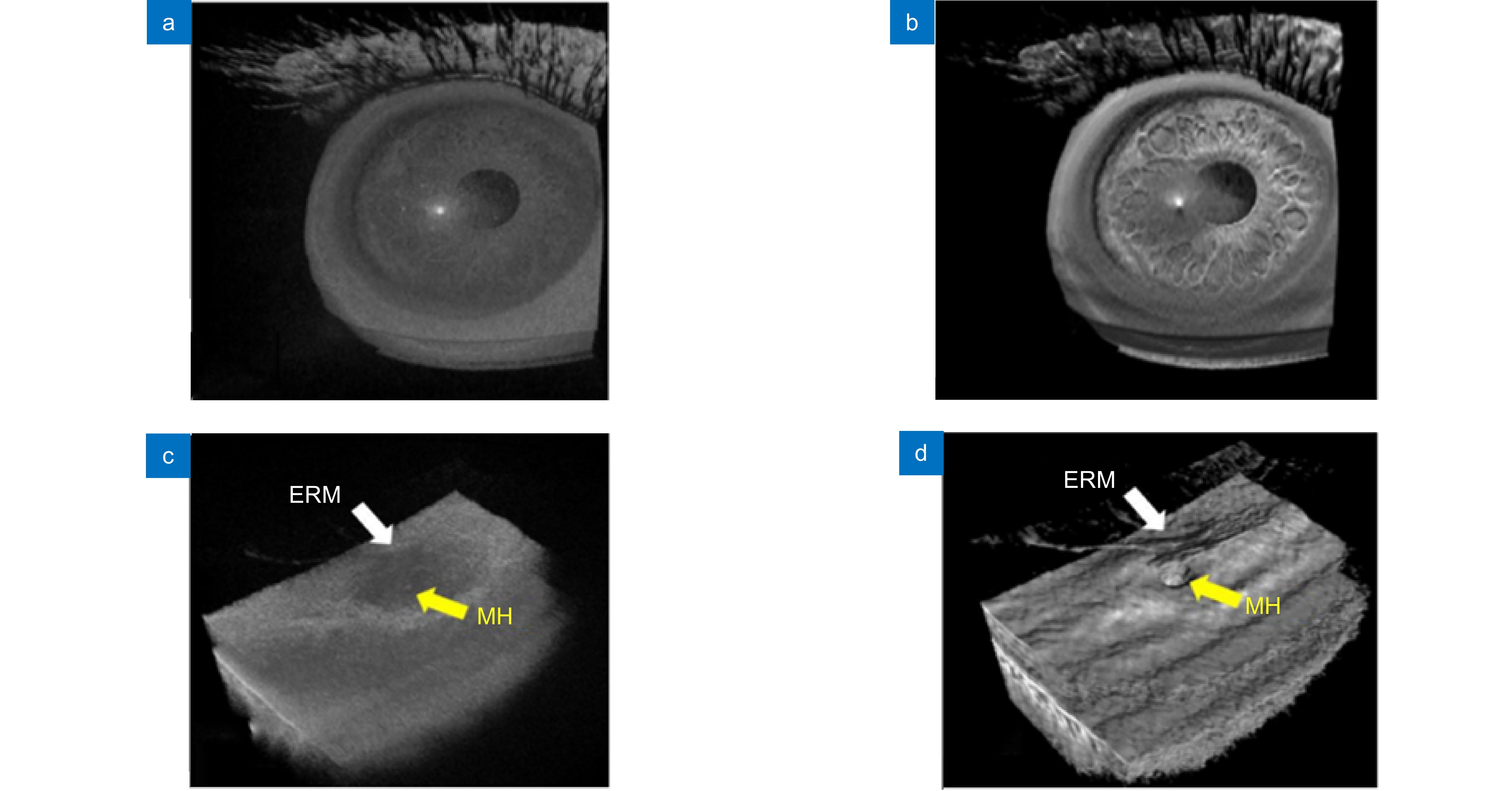
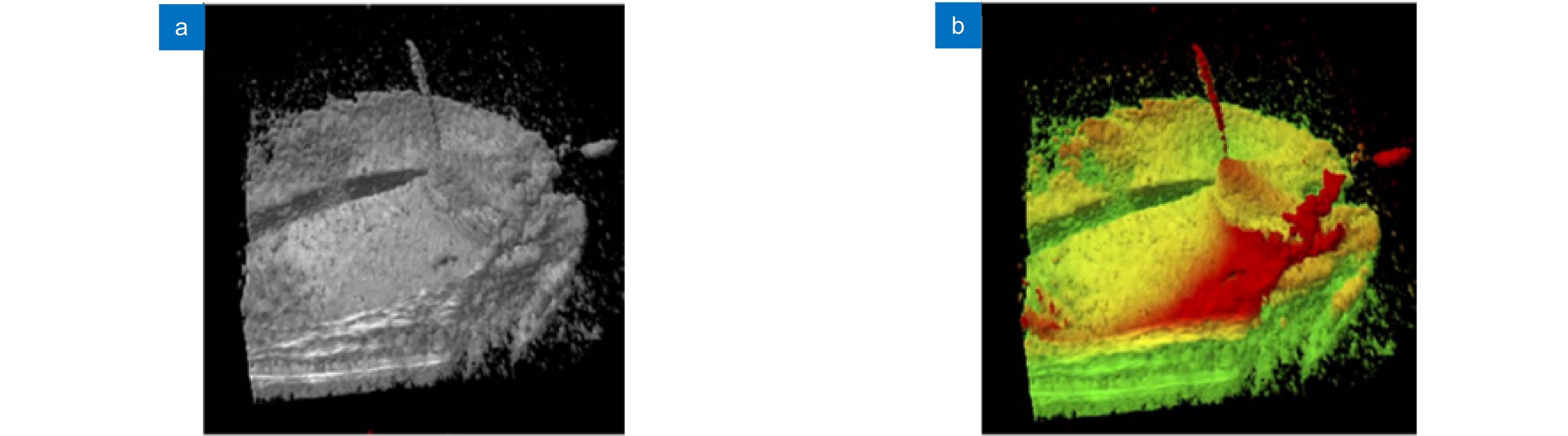
图 14 使用体积增强渲染算法处理MIOCT图像[81]。(a) 渲染前的人眼MIOCT图像;(b) 增强渲染后的人眼MIOCT图像;(c) 原始视网膜(Epiretinal membrane, ERM)及黄斑孔(macular hole, MH)MIOCT图像;(d) 增强渲染后的视网膜及黄斑孔MIOCT图像
Figure 14. MIOCT images with enhanced volume rendering[81]. (a) Original MIOCT image of human eye; (b) Enhanced MIOCT image of human eye; (c) Original MIOCT image of an epiretinal membrane (ERM) and a macular hole (MH); (d) Enhanced MIOCT image of an epiretinal membrane (ERM) and a macular hole (MH)
-
参考文献
[1] Scott A W, Farsiu S, Enyedi L B, et al. Imaging the infant retina with a hand-held spectral-domain optical coherence tomography device[J]. Am J Ophthalmol, 2009, 147(2): 364−373.e2. doi: 10.1016/j.ajo.2008.08.010
[2] Carrasco-Zevallos O M, Viehland C, Keller B, et al. Review of intraoperative optical coherence tomography: technology and applications[J]. Biomed Opt Express, 2017, 8(3): 1607−1637. doi: 10.1364/BOE.8.001607
[3] Muijzer M B, Schellekens P A W J, Beckers H J M, et al. Clinical applications for intraoperative optical coherence tomography: a systematic review[J]. Eye, 2022, 36(2): 379−391. doi: 10.1038/s41433-021-01686-9
[4] Zhang S L, Liu L W, Ren S, et al. Recent advances in nonlinear optics for bio-imaging applications[J]. Opto-Electron Adv, 2020, 3(10): 200003. doi: 10.29026/oea.2020.200003
[5] Fercher A F, Drexler W, Hitzenberger C K, et al. Optical coherence tomography-principles and applications[J]. Rep Prog Phys, 2003, 66(2): 239−303. doi: 10.1088/0034-4885/66/2/204
[6] Choma M A, Sarunic M V, Yang C, et al. Sensitivity advantage of swept source and Fourier domain optical coherence tomography[J]. Opt Express, 2003, 11(18): 2183−2189. doi: 10.1364/OE.11.002183
[7] Leitgeb R, Hitzenberger C K, Fercher A F. Performance of Fourier domain vs. time domain optical coherence tomography[J]. Opt Express, 2003, 11(8): 889−894. doi: 10.1364/OE.11.000889
[8] Drexler W, Fujimoto J G. State-of-the-art retinal optical coherence tomography[J]. Prog Retin Eye Res, 2008, 27(1): 45−88. doi: 10.1016/j.preteyeres.2007.07.005
[9] Suter M J, Tearney G J, Oh W Y, et al. Progress in intracoronary optical coherence tomography[J]. IEEE J Sel Top Quantum Electron, 2010, 16(4): 706−714. doi: 10.1109/JSTQE.2009.2035333
[10] Yoo H, Kim J W, Shishkov M, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo[J]. Nat Med, 2011, 17(12): 1680−1684. doi: 10.1038/nm.2555
[11] Gora M J, Sauk J S, Carruth R W, et al. Tethered capsule endomicroscopy enables less invasive imaging of gastrointestinal tract microstructure[J]. Nat Med, 2013, 19(2): 238−240. doi: 10.1038/nm.3052
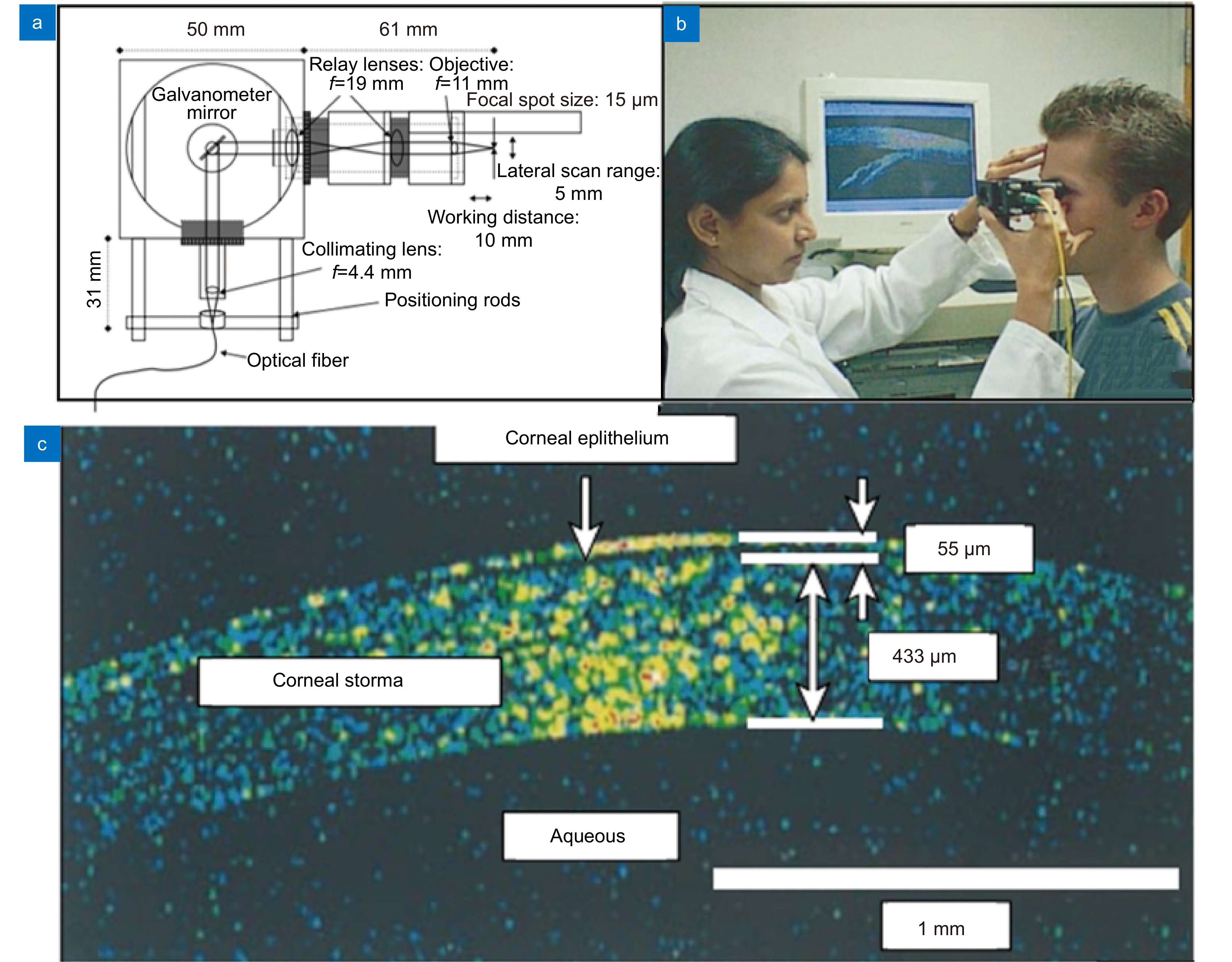
[12] Boppart S A, Luo W, Marks D L, et al. Optical coherence tomography: feasibility for basic research and image-guided surgery of breast cancer[J]. Breast Cancer Res Treat, 2004, 84(2): 85−97. doi: 10.1023/B:BREA.0000018401.13609.54
[13] Kang J Q, Zhu R, Sun Y X, et al. Pencil-beam scanning catheter for intracoronary optical coherence tomography[J]. Opto-Electron Adv, 2022, 5(3): 200050. doi: 10.29026/oea.2022.200050
[14] 刘敬璇, 樊金宇, 汪权, 等. SS-OCTA对黑色素瘤皮肤结构和血管的成像实验[J]. 光电工程, 2020, 47(2): 190239. doi: 10.12086/oee.2020.190239
Liu J X, Fan J Y, Wang Q, et al. Imaging of skin structure and vessels in melanoma by swept source optical coherence tomography angiography[J]. Opto-Electron Eng, 2020, 47(2): 190239. doi: 10.12086/oee.2020.190239
[15] Wilkins J R, Puliafito C A, Hee M R, et al. Characterization of epiretinal membranes using optical coherence tomography[J]. Ophthalmology, 1996, 103(12): 2142−2151. doi: 10.1016/S0161-6420(96)30377-1
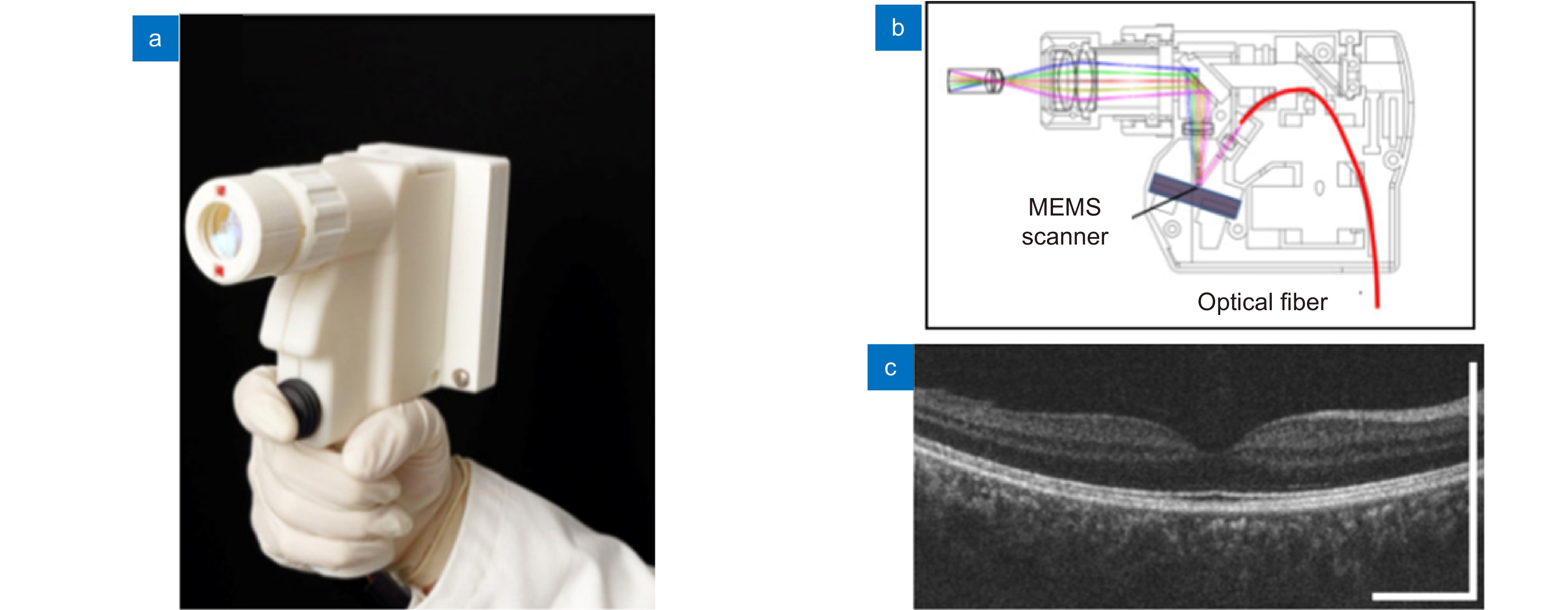
[16] Massin P, Allouch C, Haouchine B, et al. Optical coherence tomography of idiopathic macular epiretinal membranes before and after surgery[J]. Am J Ophthalmol, 2000, 130(6): 732−739. doi: 10.1016/S0002-9394(00)00574-2
[17] Kasuga Y, Arai J, Akimoto M, et al. Optical coherence tomograghy to confirm early closure of macular holes[J]. Am J Ophthalmol, 2000, 130(5): 675−676. doi: 10.1016/S0002-9394(00)00587-0
[18] Behrens A, Stark W J, Pratzer K A, et al. Dynamics of small-incision clear cornea wounds after phacoemulsification surgery using optical coherence tomography in the early postoperative period[J]. J Refract Surg, 2008, 24(1): 46−49. doi: 10.3928/1081597X-20080101-07
[19] Wei J, Hellmuth T. Optical coherence tomography assisted ophthalmologic surgical microscope: 5493109[P]. 1996-02-20.
[20] Boppart S A, Bouma B E, Pitris C, et al. Intraoperative assessment of microsurgery with three-dimensional optical coherence tomography[J]. Radiology, 1998, 208(1): 81−86. doi: 10.1148/radiology.208.1.9646796
[21] Tao Y K, Ehlers J P, Toth C A, et al. Intraoperative spectral domain optical coherence tomography for vitreoretinal surgery[J]. Opt Lett, 2010, 35(20): 3315−3317. doi: 10.1364/OL.35.003315
[22] Fujimoto J, Swanson E. The development, commercialization, and impact of optical coherence tomography[J]. Invest Ophthalmol Vis Sci, 2016, 57(9): OCT1−OCT13. doi: 10.1167/iovs.16-19963
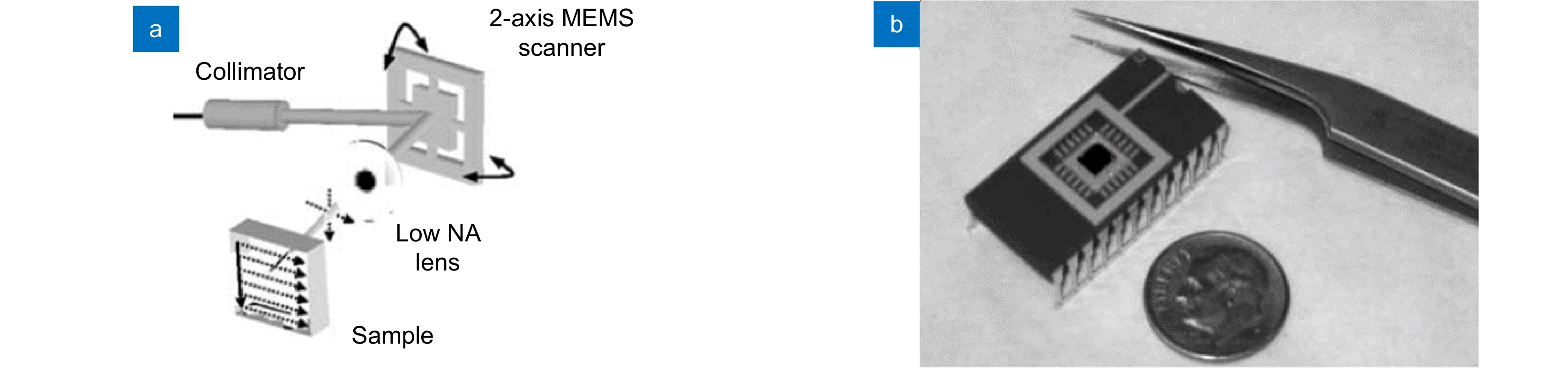
[23] Huang D, Swanson E A, Lin C P, et al. Optical coherence tomography[J]. Science, 1991, 254(5035): 1178−1181. doi: 10.1126/science.1957169
[24] Danielson B L, Boisrobert C Y. Absolute optical ranging using low coherence interferometry[J]. Appl Opt, 1991, 30(21): 2975−2979. doi: 10.1364/AO.30.002975
[25] 陆冬筱, 房文汇, 李玉瑶, 等. 光学相干层析成像技术原理及研究进展[J]. 中国光学, 2020, 13(5): 919−935. doi: 10.37188/CO.2020-0037
Lu D X, Fang W H, Li Y Y, et al. Optical coherence tomography: principles and recent developments[J]. Chin Opt, 2020, 13(5): 919−935. doi: 10.37188/CO.2020-0037
[26] Qin J, An L. Optical coherence tomography for ophthalmology imaging[M]//Wei X B, Gu B B. Optical Imaging in Human Disease and Biological Research. Singapore: Springer, 2021: 197–216. doi: 10.1007/978-981-15-7627-0_10.
[27] Szydlo J, Delachenal N, Gianotti R, et al. Air-turbine driven optical low-coherence reflectometry at 28. 6-kHz scan repetition rate[J]. Opt Commun, 1998, 154(1–3): 1−4. doi: 10.1016/S0030-4018(98)00303-4
[28] Alexopoulos P, Madu C, Wollstein G, et al. The development and clinical application of innovative optical ophthalmic imaging techniques[J]. Front Med, 2022, 9: 891369. doi: 10.3389/FMED.2022.891369
[29] Rollins A M, Kulkarni M D, Yazdanfar S, et al. In vivo video rate optical coherence tomography[J]. Opt Express, 1998, 3(6): 219−229. doi: 10.1364/OE.3.000219
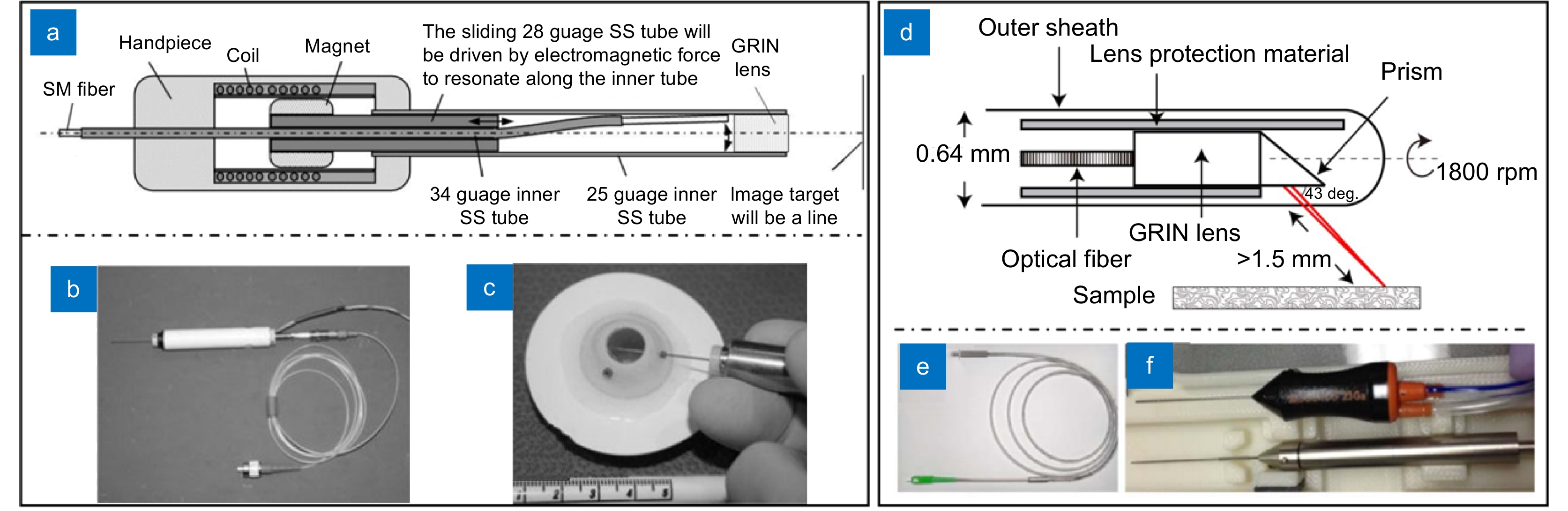
[30] 姜盼秋, 汪平河. 谱域光学相干层析系统的色散补偿技术研究[J]. 光电工程, 2021, 48(10): 210184. doi: 10.12086/oee.2021.210184
Jiang P Q, Wang P H. Research on dispersion compensation technology for SD-OCT system[J]. Opto-Electron Eng, 2021, 48(10): 210184. doi: 10.12086/oee.2021.210184
[31] Tearney G J, Bouma B E, Fujimoto J G. High-speed phase-and group-delay scanning with a grating-based phase control delay line[J]. Opt Lett, 1997, 22(23): 1811−1813. doi: 10.1364/OL.22.001811
[32] Potsaid B, Baumann B, Huang D, et al. Ultrahigh speed 1050nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100, 000 to 400, 000 axial scans per second[J]. Opt Express, 2010, 18(19): 20029−20048. doi: 10.1364/OE.18.020029
[33] Cense B, Nassif N A, Chen T C, et al. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography[J]. Opt Express, 2004, 12(11): 2435−2447. doi: 10.1364/OPEX.12.002435
[34] An L, Li P, Shen T T, et al. High speed spectral domain optical coherence tomography for retinal imaging at 500, 000 A-lines per second[J]. Biomed Opt Express, 2011, 2(10): 2770−2783. doi: 10.1364/BOE.2.002770
[35] Gora M, Karnowski K, Szkulmowski M, et al. Ultra high-speed swept source OCT imaging of the anterior segment of human eye at 200 kHz with adjustable imaging range[J]. Opt Express, 2009, 17(17): 14880−14894. doi: 10.1364/OE.17.014880
[36] Chinn S R, Swanson E A, Fujimoto J G. Optical coherence tomography using a frequency-tunable optical source[J]. Opt Lett, 1997, 22(5): 340−342. doi: 10.1364/OL.22.000340
[37] Monroy G L, Won J, Spillman Jr D R, et al. Clinical translation of handheld optical coherence tomography: practical considerations and recent advancements[J]. J Biomed Opt, 2017, 22(12): 121715. doi: 10.1117/1.JBO.22.12.121715
[38] Chen X, Tai V, McGeehan B, et al. Repeatability and reproducibility of axial and lateral measurements on handheld optical coherence tomography systems compared with tabletop system[J]. Transl Vis Sci Technol, 2020, 9(11): 25. doi: 10.1167/tvst.9.11.25
[39] Boppart S A, Bouma B E, Pitris C, et al. Forward-imaging instruments for optical coherence tomography[J]. Opt Lett, 1997, 22(21): 1618−1620. doi: 10.1364/OL.22.001618
[40] Swanson E A, Izatt J A, Hee M R, et al. In vivo retinal imaging by optical coherence tomography[J]. Opt Lett, 1993, 18(21): 1864−1866. doi: 10.1364/OL.18.001864
[41] Radhakrishnan S, Rollins A M, Roth J E, et al. Real-time optical coherence tomography of the anterior segment at 1310 nm[J]. Arch Ophthalmol, 2001, 119(8): 1179−1185. doi: 10.1001/archopht.119.8.1179
[42] Lu C D, Kraus M F, Potsaid B, et al. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror[J]. Biomed Opt Express, 2014, 5(1): 293−311. doi: 10.1364/BOE.5.000293
[43] Brown W J, Buckland E L, Izatt J A. Portable optical coherence tomography (OCT) devices and related systems: 8064989[P]. 2011-11-22.
[44] Maldonado R S, Izatt J A, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children[J]. Invest Ophthalmol Vis Sci, 2010, 51(5): 2678−2685. doi: 10.1167/iovs.09-4403
[45] Dayani P N, Maldonado R, Farsiu S, et al. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery[J]. Retina, 2009, 29(10): 1457−1468. doi: 10.1097/IAE.0b013e3181b266bc
[46] Jung W, Kim J, Jeon M, et al. Handheld optical coherence tomography scanner for primary care diagnostics[J]. IEEE Trans Biomed Eng, 2011, 58(3): 741−744. doi: 10.1109/TBME.2010.2096816
[47] Riazi-Esfahani M, Khademi M R, Mazloumi M, et al. Macular surgery using intraoperative spectral domain optical coherence tomography[J]. J Ophthalmic Vis Res, 2015, 10(3): 309. doi: 10.4103/2008-322X.170355
[48] Pichi F, Alkabes M, Nucci P, et al. Intraoperative SD-OCT in macular surgery[J]. Ophthalmic Surg Lasers Imaging, 2012, 43(S6): S54−S60. doi: 10.3928/15428877-20121001-08
[49] Yeow J T W, Yang V X D, Chahwan A, et al. Micromachined 2-D scanner for 3-D optical coherence tomography[J]. Sens Actuators A Phys, 2005, 117(2): 331−340. doi: 10.1016/j.sna.2004.06.021
[50] Jung W, Zhang J, Wang L, et al. Three-dimensional optical coherence tomography employing a 2-axis microelectromechanical scanning mirror[J]. IEEE J Sel Top Quantum Electron, 2005, 11(4): 806−810. doi: 10.1109/JSTQE.2005.857683
[51] Aguirre A D, Herz P R, Chen Y, et al. Two-axis MEMS scanning catheter for ultrahigh resolution three-dimensional and En face imaging[J]. Opt Express, 2007, 15(5): 2445−2453. doi: 10.1364/OE.15.002445
[52] Grulkowski I, Liu J J, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers[J]. Biomed Opt Express, 2012, 3(11): 2733−2751. doi: 10.1364/BOE.3.002733
[53] Grulkowski I, Liu J J, Potsaid B, et al. High-precision, high-accuracy ultralong-range swept-source optical coherence tomography using vertical cavity surface emitting laser light source[J]. Opt Lett, 2013, 38(5): 673−675. doi: 10.1364/OL.38.000673
[54] Nankivil D, Waterman G, LaRocca F, et al. Handheld, rapidly switchable, anterior/posterior segment swept source optical coherence tomography probe[J]. Biomed Opt Express, 2015, 6(11): 4516−4528. doi: 10.1364/BOE.6.004516
[55] LaRocca F, Nankivil D, DuBose T, et al. In vivo cellular-resolution retinal imaging in infants and children using an ultracompact handheld probe[J]. Nat Photonics, 2016, 10(9): 580−584. doi: 10.1038/nphoton.2016.141
[56] Asami T, Terasaki H, Ito Y, et al. Development of a fiber-optic optical coherence tomography probe for intraocular use[J]. Invest Ophthalmol Vis Sci, 2016, 57(9): OCT568−OCT574. doi: 10.1167/iovs.15-18853
[57] Joos K M, Shen J H. Miniature real-time intraoperative forward-imaging optical coherence tomography probe[J]. Biomed Opt Express, 2013, 4(8): 1342−1350. doi: 10.1364/BOE.4.001342
[58] Song C, Park D Y, Gehlbach P L, et al. Fiber-optic OCT sensor guided “SMART” micro-forceps for microsurgery[J]. Biomed Opt Express, 2013, 4(7): 1045−1050. doi: 10.1364/BOE.4.001045
[59] Cheon G W, Huang Y, Cha J, et al. Accurate real-time depth control for CP-SSOCT distal sensor based handheld microsurgery tools[J]. Biomed Opt Express, 2015, 6(5): 1942−1953. doi: 10.1364/BOE.6.001942
[60] Song C, Gehlbach P L, Kang J U. Active tremor cancellation by a “smart” handheld vitreoretinal microsurgical tool using swept source optical coherence tomography[J]. Opt Express, 2012, 20(21): 23414−23421. doi: 10.1364/OE.20.023414
[61] Yu H R, Shen J H, Shah R J, et al. Evaluation of microsurgical tasks with OCT-guided and/or robot-assisted ophthalmic forceps[J]. Biomed Opt Express, 2015, 6(2): 457−472. doi: 10.1364/BOE.6.000457
[62] Binder S, Falkner-Radler C I, Hauger C, et al. Feasibility of intrasurgical spectral-domain optical coherence tomography[J]. Retina, 2011, 31(7): 1332−1336. doi: 10.1097/IAE.0b013e3182019c18
[63] Posarelli C, Sartini F, Casini G, et al. What is the impact of intraoperative microscope-integrated OCT in ophthalmic surgery? Relevant applications and outcomes. A systematic review[J]. J Clin Med, 2020, 9(6): 1682. doi: 10.3390/jcm9061682
[64] Lankenau E, Klinger D, Winter C, et al. Combining optical coherence tomography (OCT) with an operating microscope[C]//Advances in Medical Engineering, 2007: 343–348. doi: 10.1007/978-3-540-68764-1_57.
[65] Ehlers J P. Intraoperative optical coherence tomography: past, present, and future[J]. Eye, 2016, 30(2): 193−201. doi: 10.1038/eye.2015.255
[66] Geerling G, Müller M, Winter C, et al. Intraoperative 2-dimensional optical coherence tomography as a new tool for anterior segment surgery[J]. Arch Ophthalmol, 2005, 123(2): 253−257. doi: 10.1001/archopht.123.2.253
[67] Probst J, Hillmann D, Lankenau E M, et al. Optical coherence tomography with online visualization of more than seven rendered volumes per second[J]. J Biomed Opt, 2010, 15(2): 026014. doi: 10.1117/1.3314898
[68] Tao Y K, Srivastava S K, Ehlers J P. Microscope-integrated intraoperative OCT with electrically tunable focus and heads-up display for imaging of ophthalmic surgical maneuvers[J]. Biomed Opt Express, 2014, 5(6): 1877−1885. doi: 10.1364/BOE.5.001877
[69] Ehlers J P, Kaiser P K, Srivastava S K. Intraoperative optical coherence tomography using the RESCAN 700: preliminary results from the DISCOVER study[J]. Br J Ophthalmol, 2014, 98(10): 1329−1332. doi: 10.1136/bjophthalmol-2014-305294
[70] Ehlers J P, Modi Y S, Pecen P E, et al. The DISCOVER study 3-year results: feasibility and usefulness of microscope-integrated intraoperative OCT during ophthalmic surgery[J]. Ophthalmology, 2018, 125(7): 1014−1027. doi: 10.1016/j.ophtha.2017.12.037
[71] Tuifua T S, Sood A B, Abraham J R, et al. Epiretinal membrane surgery using intraoperative OCT-guided membrane removal in the DISCOVER study versus conventional membrane removal[J]. Ophthalmol Retina, 2021, 5(12): 1254−1262. doi: 10.1016/j.oret.2021.02.013
[72] Hahn P, Carrasco-Zevallos O, Cunefare D, et al. Intrasurgical human retinal imaging with manual instrument tracking using a microscope-integrated spectral-domain optical coherence tomography device[J]. Transl Vis Sci Technol, 2015, 4(4): 1. doi: 10.1167/tvst.4.4.1
[73] Ehlers J P, Goshe J, Dupps W J, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER study RESCAN results[J]. JAMA Ophthalmol, 2015, 133(10): 1124−1132. doi: 10.1001/jamaophthalmol.2015.2376
[74] Zhang K, Kang J U. Real-time intraoperative 4D full-range FD-OCT based on the dual graphics processing units architecture for microsurgery guidance[J]. Biomed Opt Express, 2011, 2(4): 764−770. doi: 10.1364/BOE.2.000764
[75] Kang J U, Huang Y, Cha J, et al. Real-time three-dimensional Fourier-domain optical coherence tomography video image guided microsurgeries[J]. J Biomed Opt, 2012, 17(8): 081403. doi: 10.1117/1.JBO.17.8.081403
[76] Li X Q, Wei L, Dong X C, et al. Microscope-integrated optical coherence tomography for image-aided positioning of glaucoma surgery[J]. J Biomed Opt, 2015, 20(7): 076001. doi: 10.1117/1.JBO.20.7.076001
[77] Fang W Y, Li Q C, Fan J Y, et al. Microscope-integrated intraoperative optical coherence tomography for anterior segment surgical maneuvers[J]. Transl Vis Sci Technol, 2020, 9(7): 18. doi: 10.1167/tvst.9.7.18
[78] Xu H, Fang W Y, Liu G X, et al. Feasibility of microscope-integrated swept-source optical coherence tomography in canaloplasty[J]. Ann Transl Med, 2020, 8(23): 1577. doi: 10.21037/ATM-20-3469
[79] Shen L B, Carrasco-Zevallos O, Keller B, et al. Novel microscope-integrated stereoscopic heads-up display for intrasurgical optical coherence tomography[J]. Biomed Opt Express, 2016, 7(5): 1711−1726. doi: 10.1364/BOE.7.001711
[80] Carrasco-Zevallos O M, Keller B, Viehland C, et al. Optical coherence tomography for retinal surgery: perioperative analysis to real-time four-dimensional image-guided surgery[J]. Invest Ophthalmol Vis Sci, 2016, 57(9): OCT37−OCT50. doi: 10.1167/iovs.16-19277
[81] Viehland C, Keller B, Carrasco-Zevallos O M, et al. Enhanced volumetric visualization for real time 4D intraoperative ophthalmic swept-source OCT[J]. Biomed Opt Express, 2016, 7(5): 1815−1829. doi: 10.1364/BOE.7.001815
[82] Hsu S T, Gabr H, Viehland C, et al. Volumetric measurement of subretinal blebs using microscope-integrated optical coherence tomography[J]. Transl Vis Sci Technol, 2018, 7(2): 19. doi: 10.1167/tvst.7.2.19
[83] Vajzovic L, Sleiman K, Viehland C, et al. Four-dimensional microscope-integrated optical coherence tomography guidance in a model eye subretinal surgery[J]. Retina, 2019, 39(S1): S194−S198. doi: 10.1097/IAE.0000000000002518
[84] Gabr H, Chen X, Zevallos-Carrasco O M, et al. Visualization from intraoperative swept-source microscope-integrated optical coherence tomography in vitrectomy for complications of proliferative diabetic retinopathy[J]. Retina, 2018, 38(S1): S110−S120. doi: 10.1097/IAE.0000000000002021
[85] Pfau M, Michels S, Binder S, et al. Clinical experience with the first commercially available intraoperative optical coherence tomography system[J]. Ophthalmic Surg Lasers Imaging Retina, 2015, 46(10): 1001−1008. doi: 10.3928/23258160-20151027-03
[86] Ehlers J P, Srivastava S K, Feiler D, et al. Integrative advances for OCT-guided ophthalmic surgery and intraoperative OCT: microscope integration, surgical instrumentation, and heads-up display surgeon feedback[J]. PLoS One, 2014, 9(8): e105224. doi: 10.1371/journal.pone.0105224
[87] Bleicher I D, Jackson-Atogi M, Viehland C, et al. Depth-based, motion-stabilized colorization of microscope-integrated optical coherence tomography volumes for microscope-independent microsurgery[J]. Transl Vis Sci Technol, 2018, 7(6): 1. doi: 10.1167/tvst.7.6.1
[88] Zhao H X, Li K, Yang F, et al. Customized anterior segment photoacoustic imaging for ophthalmic burn evaluation in vivo[J]. Opto-Electron Adv, 2021, 4(6): 200017. doi: 10.29026/oea.2021.200017
-
访问统计


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: