Design of high-resolution wide field of view confocal line scanning laser microscopy
-
摘要
商用共聚焦显微镜使用二维光点扫描对样品成像,成像帧频被限制在30 Hz以内,扫描速度大多在10帧/秒(f/s)左右。为了提高共聚焦系统成像速度,满足生物细胞在体成像的需求,本文使用线光束对样品进行一维扫描照明,成像速度大大提高,同时根据共聚焦成像原理,在线阵CCD前使用狭缝滤除非聚焦平面杂散光以提高成像质量。实验表明,系统光学放大倍率为55倍,横向分辨率高于2.2 μm,当线阵CCD以28 kHz行频扫描成像时,帧频可达50 f/s,通过对动植物细胞成像证明,本系统可用于生物细胞的在体成像。

Abstract
Abstract: Commercial confocal microscopy usually utilizes two-dimensional galvanometer to scan specimen, the frame rate is limited within 30 f/s, and commercial confocal microscopy is 10 f/s or lower. In order to improve the imaging speed of confocal system, and meet the needs of real-time observation of in vivo imaging, in the high-speed confocal line scanning laser microscopy, the sample is illuminated by one-dimensional scanning laser beam. The imaging speed is greatly improved. At the same time, a slit is placed before the linear array CCD to filter out to the non-focused plane stray light and improve the image quality. Experiments show that the optical magnification is 55, horizontal resolution is higher than 2.2 μm, and the frame rate is up to 50 frame per second when the linear CCD scans with 28 kHz horizontal frequency and 512 pixelsx2048 pixels image resolution, image experiment of plant and animal cells prove that this imaging system could be used in ex vivo or in vivo cell image.
-
Key words:
- imaging system /
- line scanning /
- confocal /
- high-frame-frequency imaging
-
Overview

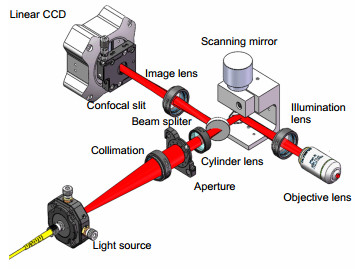
In traditional commercial confocal image system, detecting light emitted from the light source through apinhole into a point light, the reflected light from specimen go through the pinhole into the detector. In this case, onlythe reflected light from the focusing plane could reach the detector. Non-focused light cannot pass through the pinhole and therefore cannot be imaged in the detector. However, traditional confocal imaging system use point by pointscanning to image the sample, so the field of view is small (200 μm200 μm), the imaging speed is slow (10 fps typical),and the image speed is much slower for larger field of view.
In order to improve the imaging speed and increase the field of view, the line scanning confocal systems useone-dimensional-focused line beam to scan the sample, and use the slit to filter light strayed from non-focused plane.Non-focused light cannot pass through the slit filter and be imaged by the detector. Compared with the point by pointscanning system, the line scanning microscope system can image the sample by only one dimension scanning, whichimproves the image speed and field of view greatly.
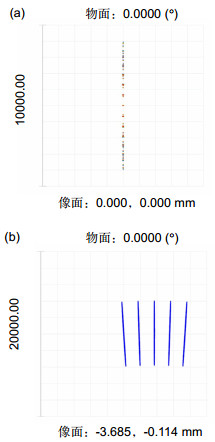
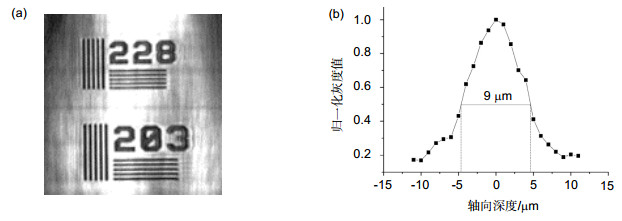
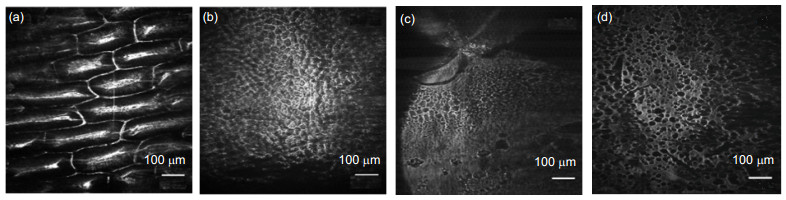
The line scanning confocal microscopes uses high speed galvanometer scanning mirror and 28 kHz line array camera to get high resolution (512 pixels 2048 pixels) image, and the frame frequency of the system could reach 50 fps(frame per second), which is much higher than the point scanning confocal microscope. And the higher line frequency the camera has, the higher imaging speed could the system reaches. Theoretical analysis and experimental resultshow that the optical magnification of the line scanning confocal microscope is 55, and the field of view is 713μm713 μm. We use resolution test target to find that the system could distinguish at least 288 line pairs in the target,which means that the lateral resolution of the line scanning confocal microscope is higher than 2.2 μm. The axial resolution of the microscope is defined as the FWHM (full width at half maximum) of the detected light intensity. Theground glass flat is placed as sample and the axial resolution is about 9 μm in this system. Finally, images of plants andhuman cells is got by the confocal line scanning microscope, and cells could clearly distinguish from the images,which proves that the system could be used in cells biological cell imaging.
-

-
-
参考文献
[1] Husebye H, Doyle S L. Using confocal microscopy to investigate intracellular trafficking of toll-like receptors[J]. Methods in Molecular Biology, 2016, 1390: 65–77. doi: 10.1007/978-1-4939-3335-8
[2] Castillo-Badillo J A, Cabrera-Wrooman A, García-Sáinz J A. Visualizing G Protein-coupled receptors in action through confocal microscopy techniques[J]. Archives of Medical Research, 2014, 45(4): 283–293. doi: 10.1016/j.arcmed.2014.03.009
[3] Shang Li, Yang Linxiao, Seiter J, et al. Nanoparticles interacting with proteins and cells: a systematic study of protein surface charge effects[J]. Advanced Materials Interfaces, 2014, 1(2): 1300079. doi: 10.1002/admi.201300079
[4] Liang Rongguang. Biomedical optical imaging technologies: design and applications[M]. Berlin Heidelberg: Springer, 2012: 221–226.
[5] 赵维谦, 任利利, 盛忠, 等.激光共焦显微光束的偏转扫描[J].光学精密工程, 2016, 24(6): 1257–1263. http://www.eope.net/gxjmgc/CN/abstract/abstract16416.shtml
Zhao Weiqian, Ren Lili, Sheng Zhong, et al. Beam deflection scanning for laser confocal microscopy[J]. Optics and Precision Engineering, 2016, 24(6): 1257–1263. http://www.eope.net/gxjmgc/CN/abstract/abstract16416.shtml
[6] 熊大曦, 刘云, 梁永, 等.共振扫描显微成像中的图像畸变校正[J].光学精密工程, 2015, 23(10): 2971–2979. http://www.eope.net/gxjmgc/article/2015/2015-10-2971.htm
Xiong Daxi, Liu Yun, Liang Yong, et al. Correction of distortion in microscopic imaging with resonant scanning[J].Optics and Precision Engineering, 2015, 23(10): 2971–2979. http://www.eope.net/gxjmgc/article/2015/2015-10-2971.htm
[7] Li Zhaohui. Design and optimization in constructing an in-vivo confocal laser scanning microscopy[J]. Acta Photonica Sinica, 2011, 40(5): 667–672. doi: 10.3788/gzxb
[8] Pircher M, Hitzenberger C K. Acousto optic modulation based en face AO SLO OCT[M] // Drexler W, Fujimoto J G. Optical Coherence Tomography: Technology and Applications. Switzerland: Springer International Publishing, 2015: 1921–1939.
[9] Zeng Shaoqun, Bi Kun, Xue Songchao, et al. Acousto-optic modulator system for femtosecond laser pulses[J]. Review of Scientific Instruments, 2007, 78(1): 015103. doi: 10.1063/1.2409868
[10] Yin C, Glaser A K, Leigh S Y, et al. Miniature in vivo MEMS-based line-scanned dual-axis confocal microscope for point-of-care pathology[J]. Biomedical Optics Express, 2016, 7(2): 251–263. doi: 10.1364/BOE.7.000251
[11] Brown C M, Dalal R B, Hebert B, et al. Raster image correlation spectroscopy (RICS) for measuring fast protein dynamics and concentrations with a commercial laser scanning confocal microscope[J].Journal of Microscopy, 2008, 229(1): 78–91. doi: 10.1111/jmi.2008.229.issue-1
[12] Moens P D J, Gratton E, Salvemini I L. Fluorescence correlation spectroscopy, raster image correlation spectroscopy, and number and brightness on a commercial confocal laser scanning microscope with analog detectors (Nikon C1)[J]. Microscopy Research & Technique, 2011, 74(4): 377–388. https://www.researchgate.net/publication/227782807_Fluorescence_correlation_spectroscopy_raster_image_correlation_spectroscopy_and_number_and_brightness_on_a_commercial_confocal_laser_scanning_microscope_with_analog_detectors_Nikon_C1
[13] Choi S H, Kim W H, Lee Y J, et al. Visualization of epidermis and dermal cells in ex vivo human skin using the confocal and two-photon microscopy[J]. Journal of the Optical Society of Korea, 2011, 15(1): 61–67. doi: 10.3807/JOSK.2011.15.1.061
[14] Chen Ye, Wang Danni, Khan A, et al. Video-rate in vivo fluorescence imaging with a line-scanned dual-axis confocal microscope[J]. Journal of Biomedical Optics, 2015, 20(10): 106011. doi: 10.1117/1.JBO.20.10.106011
[15] Choi S M, Kim W H, Côté D, et al. Blood cell assisted in vivo particle image velocimetry using the confocal laser scanning microscope[J]. Optics Express, 2011, 19(5): 4357–4368. doi: 10.1364/OE.19.004357
[16] B hnke M, Masters B R. Confocal microscopy of the cornea[J]. Progress in Retinal and Eye Research, 1999, 18(5): 553–628. doi: 10.1016/S1350-9462(98)00028-7
[17] 何益, 史国华, 卢婧, 等.高速线扫描共焦检眼镜[J].光学学报, 2012, 32(1): 0117001. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxxb201201029&dbname=CJFD&dbcode=CJFQ
He Yi, Shi Guohua, Lu Jing, et al. High-speed line scanning confocal laser ophthalmoscope[J]. Acta Optica Sinica, 2012, 32(1): 0117001. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxxb201201029&dbname=CJFD&dbcode=CJFQ
[18] Dwyer P J, DiMarzio C A, Rajadhyaksha M. Confocal theta line-scanning microscope for imaging human tissues[J]. Applied Optics, 2007, 46(10): 1843–1851. doi: 10.1364/AO.46.001843
-
访问统计


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: