Assessment of optic nerve injury with polarization-sensitive optical coherence tomography
-
摘要:
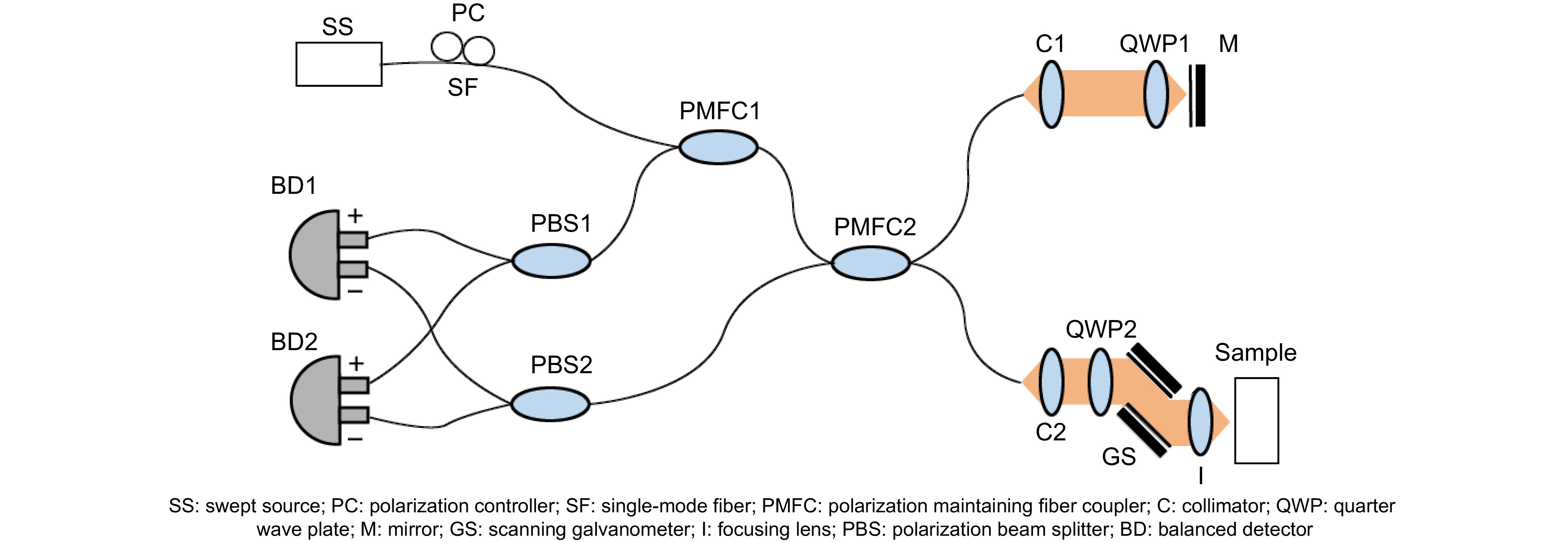
视神经损伤是视力丧失的主要原因之一,准确评估视神经损伤程度对相关疾病的有效治疗和康复至关重要。本文利用基于保偏光纤元器件构建的扫频偏振敏感光学相干层析系统,对损伤前后的离体猪眼视神经成像,观察视神经内的微观结构,并通过探测光偏振态的斯托克斯参数
Q 、U 、V 反映视神经组织内的双折射特性。发现V 横截面图对视神经的双折射特性具有较好的表征能力,通过阈值法对V 横截面图对应的高双折射区域和无双折射或低双折射区域进行分割。V 横截面图中的高双折射区域平均面积和平均高度的演化与视神经的损伤、修复和糜烂存在一定的对应关系。实验表明,偏振敏感光学相干层析对视神经损伤前后的变化具有较好的感知能力,对视神经损伤程度的评估至关重要,可以为视神经损伤的早期诊断和治疗提供重要参考数据。-
关键词:
- 偏振敏感光学相干层析 /
- 视神经损伤 /
- 神经纤维 /
- 双折射
Abstract:Optic nerve injury is one of the primary causes of vision loss, so accurately assessing the extent of optic nerve fiber damage is critically important for effective treatment and rehabilitation. In this manuscript, the optic nerves of pig eyes are imaged before and after injuries using a swept-source polarization-sensitive optical coherence tomography system built with polarization-maintaining fiber components. The microstructure and birefringence characteristics of the optic nerve are observed and reflected through Stokes parameters
Q ,U , andV , representing the polarization state of the detection light. It was found that theV cross-sectional image has good characterization ability for the birefringence characteristics of the optic nerve. The high birefringence region and the non-birefringence or low birefringence region corresponding to theV cross-sectional image were segmented by the threshold method. The evolution of the average area and the average height of the high birefringence region in the cross-sectional image ofV can reflect the damage, repair, and erosion of the optic nerve, which indicates that polarization-sensitive optical coherence tomography has a good perceptual ability for changes before and after optic nerve injury and is crucial for evaluating the degree of optic nerve injury, which can provide important reference data for early diagnosis and treatment of optic nerve injury. -
Overview: Optic nerve injury is one of the foremost causes of vision loss. Thus, the precise and meticulous assessment of the extent of optic nerve fiber damage is essential for efficacious treatment and rehabilitation. A laboratory-fabricated swept-source polarization-sensitive optical coherence tomography (PS-OCT) system, assembled with polarization-maintaining fiber optic components, was utilized to capture imaging of the optic nerves in porcine eyes before and after the infliction of injuries. The PS-OCT imaging technique allowed for the acquisition of microstructural details and polarization properties of the optic nerve tissue. The birefringent characteristics of the optic nerve tissue were elucidated through the polarization state of the probing light in the PS-OCT system. They were visualized using Stokes parameters Q, U, and V. It was discovered that the V cross-sectional image demonstrated superior capabilities in representing the birefringent properties of the optic nerve. Through the application of a threshold segmentation methodology, the V cross-sectional images were utilized to separate high-birefringence regions from non-birefringence or low-birefringence regions. The nerve fiber tissue exhibiting high birefringence corresponded to blue areas within the cross-sectional images, which significantly contrasted with the background color. Prior to optic nerve injury, the blue areas in the V cross-sectional images occupied the largest area. After injury, the area of the blue regions in the V cross-sectional images decreased abruptly. As the duration post-injury progressed, the necrosis of cells and tissue degradation led to an increase in scattering effects, causing a gradual overall intensification of the signal in the cross-sectional structural images. In the V cross-sectional images taken at 0.5- and 1.0 hours post-injury, there was a partial rebound in the blue areas. However, at the 2-hour mark, the area of the blue regions diminished once again. The evolving pattern of the average thickness and area of the nerve fibers corresponding to the blue regions in the V cross-sectional images followed a consistent trend, presenting an inverted “N” shape, which appeared to correlate with nerve injury, repair, and degeneration processes, which strongly indicates that the information regarding the changes in fiber structure and polarization characteristics of the optic nerve obtained through PS-OCT is critically important for assessing the severity of optic nerve damage. The progressive changes in fiber structure revealed by this imaging technique provide crucial reference data for the early diagnosis and therapeutic intervention in cases of optic nerve injury.
-

-
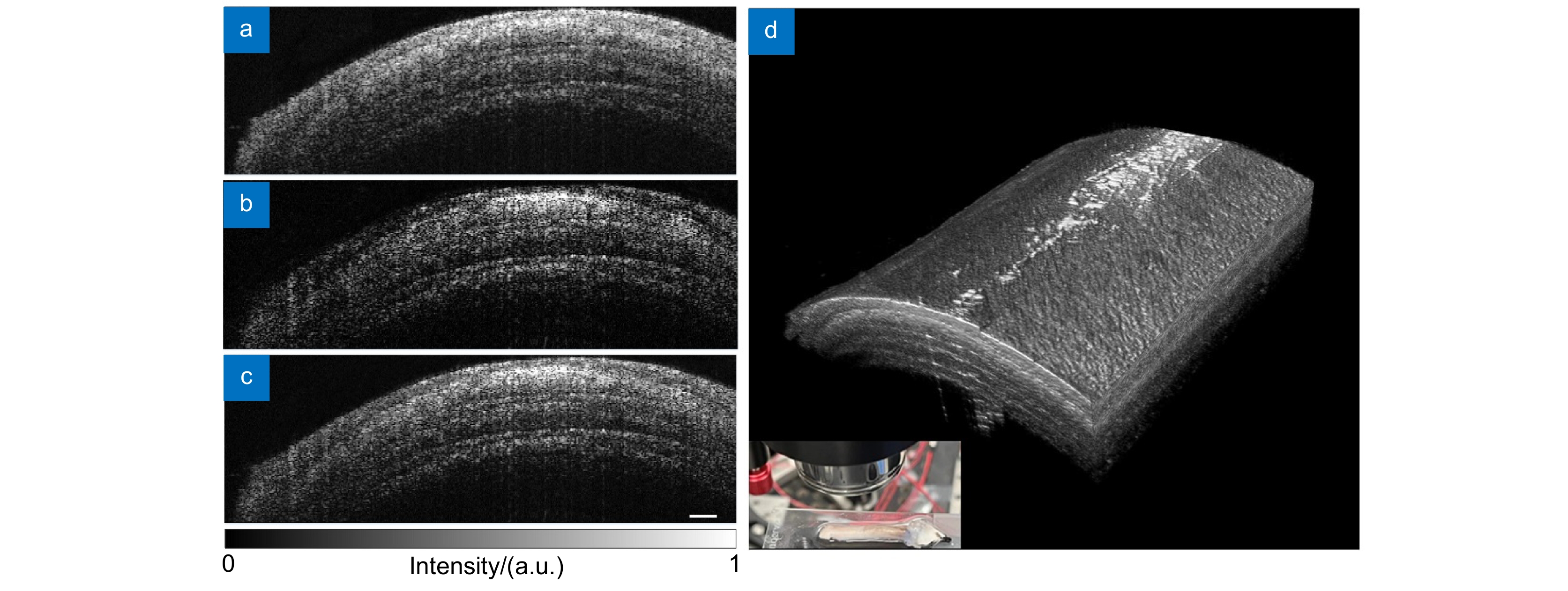
图 2 离体视神经损伤前结构图。(a)慢轴通道横截面结构图;(b)快轴通道横截面结构图;(c)合成通道横截面结构图;(d)三维结构图。标尺为200 μm
Figure 2. Structure images of ex vivo optic nerve before injury. (a) Cross-sectional structure image of slow axis channel; (b) Cross-sectional structure image of fast axis channel; (c) Cross-sectional structure image of dual-channel composition; (d) 3D structure image. Scale bar of 200 μm
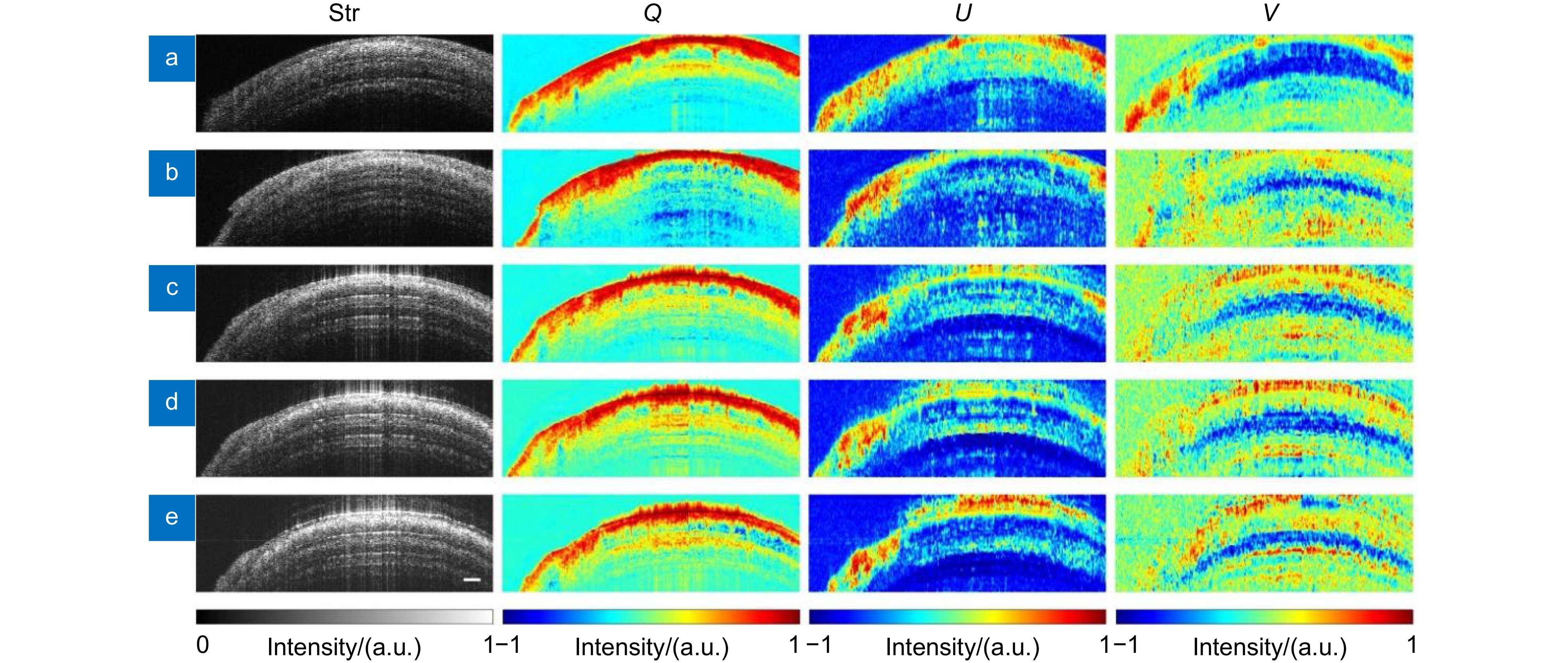
图 3 视神经损伤前后不同时间点离体视神经结构和斯托克斯矢量元素Q、U、V横截面图。(a)损伤前; (b)损伤后即时;(c)损伤后0.5 h;(d)损伤后1.0 h;(e)损伤后2.0 h
Figure 3. Cross-sectional structure and Stokes vector elements Q, U, V images of the ex vivo optic nerve at different time points before and after injury. (a) Before injury; (b) Immediately after injury; (c) 0.5 hours after injury; (d) 1.0 hours after injury; (e) 2.0 hours after injury
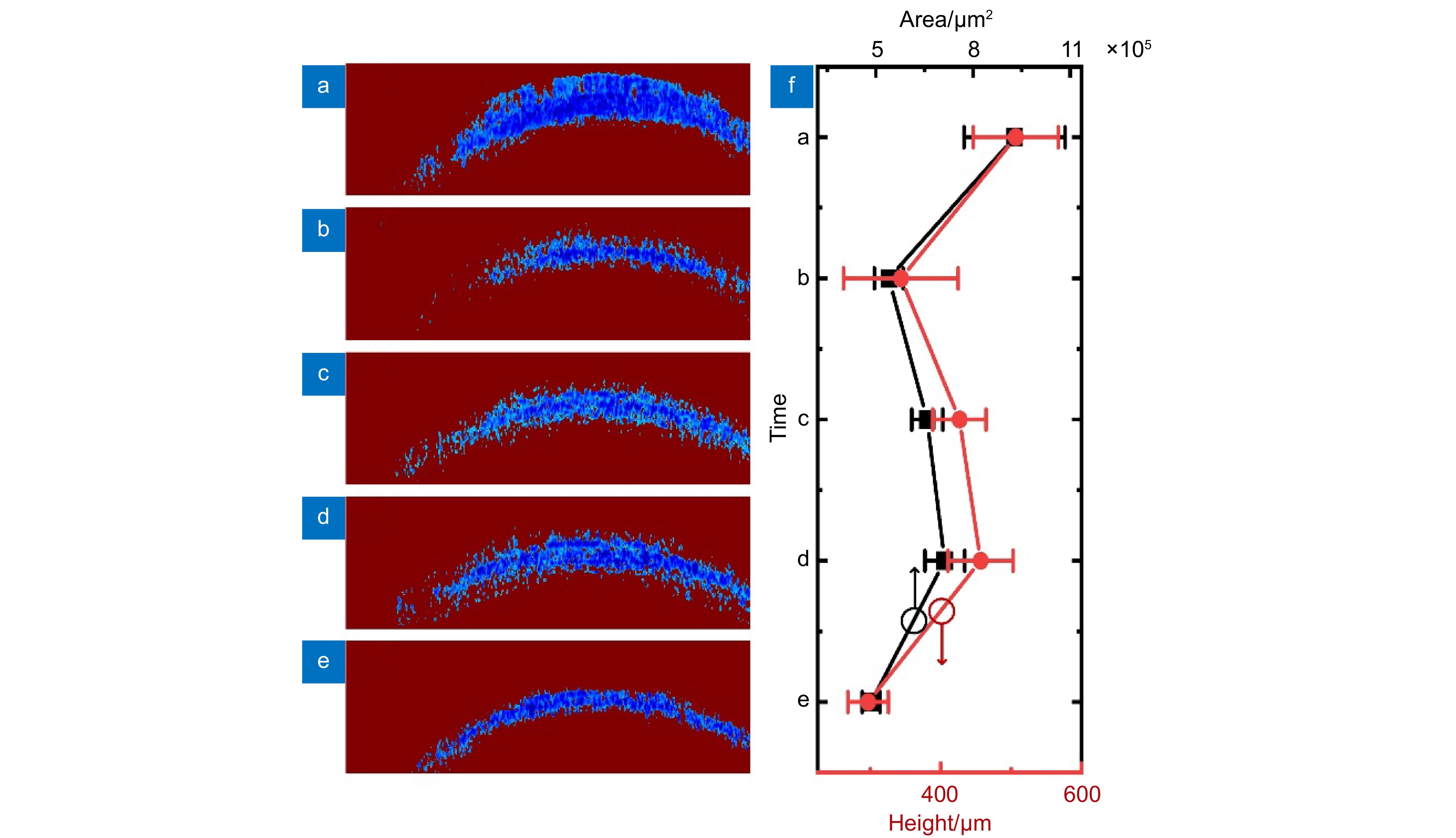
图 4 分割后的视神经损伤前后不同时间点斯托克斯矢量元素V横截面图。(a)损伤前;(b)损伤后即时;(c)损伤后0.5 h;(d)损伤后1.0 h;(e)损伤后2.0 h;(f) V横截面图中非1元素对应面积和高度的平均值及标准差在视神经损伤前后不同时间点的演化
Figure 4. Segmented cross-sectional Stokes vector element V images of the optic nerve at different time points before and after injury. (a) Before injury; (b) Immediately after injury; (c) 0.5 hours after injury; (d) 1.0 hours after injury; (e) 2.0 hours after injury; (f) The evolution of the average and the standard deviation of the area and height corresponding to non-1 elements in the cross-sectional images of V at different time before and after optic nerve injury
-
[1] Selhorst J B, Chen Y J. The optic nerve[J]. Semin Neurol, 2009, 29 (1): 29−35. doi: 10.1055/s-0028-1124020
[2] Becker M, Masterson K, Delavelle J, et al. Imaging of the optic nerve[J]. Eur J Radiol, 2010, 74 (2): 299−313. doi: 10.1016/j.ejrad.2009.09.029
[3] Hosseini Siyanaki M R, Azab M A, Lucke-Wold B. Traumatic optic neuropathy: update on management[J]. Encyclopedia, 2023, 3 (1): 88−101. doi: 10.3390/encyclopedia3010007
[4] 隗雨, 马琼, 王常科, 等. 基因编辑技术在视神经再生修复中的应用[J]. 激光生物学报, 2023, 32 (5): 414−422. doi: 10.3969/j.issn.1007-7146.2023.05.004
Wei Y, Ma Q, Wang C K, et al. Advances in optic nerve regenerative repair signaling pathways and gene editing therapeutics[J]. Acta Laser Biol Sin, 2023, 32 (5): 414−422. doi: 10.3969/j.issn.1007-7146.2023.05.004
[5] Benowitz L I, He Z G, Goldberg J L. Reaching the brain: advances in optic nerve regeneration[J]. Exp Neurol, 2017, 287 (Pt 3): 365−373. doi: 10.1016/j.expneurol.2015.12.015
[6] Gospe III S M, Chen J J, Bhatti M T. Neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein associated disorder-optic neuritis: a comprehensive review of diagnosis and treatment[J]. Eye (Lond), 2021, 35 (3): 753−768. doi: 10.1038/s41433-020-01334-8
[7] Huang D, Swanson E A, Lin C P, et al. Optical coherence tomography[J]. Science, 1991, 254 (5035): 1178−1181. doi: 10.1126/science.1957169
[8] Laíns I, Wang J C, Cui Y, et al. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA)[J]. Prog Retin Eye Res, 2021, 84: 100951. doi: 10.1016/j.preteyeres.2021.100951
[9] Eladawi N, Elmogy M, Ghazal M, et al. Classification of retinal diseases based on OCT Images[J]. Front Biosci (Landmark Ed), 2018, 23 (2): 247−264. doi: 10.2741/4589
[10] Lamirel C, Newman N J, Biousse V. Optical coherence tomography (OCT) in optic neuritis and multiple sclerosis[J]. Rev Neurol (Paris), 2010, 166 (12): 978−986. doi: 10.1016/j.neurol.2010.03.024
[11] Garcia-Martin E, Pinilla I, Sancho E, et al. Optical coherence tomography in retinitis pigmentosa: reproducibility and capacity to detect macular and retinal nerve fiber layer thickness alterations[J]. Retina, 2012, 32 (8): 1581−1591. doi: 10.1097/IAE.0b013e318242b838
[12] Li Z S, Sun J S, Fan Y, et al. Deep learning assisted variational Hilbert quantitative phase imaging[J]. Opto-Electron Sci, 2023, 2: 220023. doi: 10.29026/oes.2023.220023
[13] Christopher M, Bowd C, Belghith A, et al. Deep learning approaches predict glaucomatous visual field damage from OCT optic nerve head En face images and retinal nerve fiber layer thickness maps[J]. Ophthalmology, 2020, 127 (3): 346−356. doi: 10.1016/j.ophtha.2019.09.036
[14] Wagner S K, Romero-Bascones D, Cortina-Borja M, et al. Retinal optical coherence tomography features associated with incident and prevalent Parkinson disease[J]. Neurology, 2023, 101 (16): e1581−e1593. doi: 10.1212/WNL.0000000000207727
[15] Hee M R, Huang D, Swanson E A, et al. Polarization-sensitive low-coherence reflectometer for birefringence characterization and ranging[J]. J Opt Soc Am B, 1992, 9 (6): 903−908. doi: 10.1364/JOSAB.9.000903
[16] 张玉荣, 常颖, 高万荣. 偏振敏感光学相干层析成像系统中样品光偏振对样品双折射相位延迟测量的影响[J]. 光学学报, 2019, 39 (12): 1212007. doi: 10.3788/AOS201939.1212007
Zhang Y R, Chang Y, Gao W R. Effect of light polarization state on phase delay measurement induced by tissue birefringence in polarization-sensitive optical coherence tomography imaging system[J]. Acta Opt Sin, 2019, 39 (12): 1212007. doi: 10.3788/AOS201939.1212007
[17] 胡燕赵, 高万荣. 偏振敏感及强度双通道光学相干层析成像方法研究[J]. 中国激光, 2024, 51 (15): 1507105. doi: 10.3788/CJL231555
Hu Y Z, Gao W R. Polarization-sensitive and intensity dual-channel optical coherence tomographic method[J]. Chin J Lasers, 2024, 51 (15): 1507105. doi: 10.3788/CJL231555
[18] Hund S M M, Golde J, Tetschke F, et al. Polarization-sensitive optical coherence tomography for monitoring de- and remineralization of bovine enamel in vitro[J]. Diagnostics (Basel), 2024, 14 (4): 367. doi: 10.3390/diagnostics14040367
[19] Xu J J, Zhu M T, Tang P J, et al. Visualization enhancement by PCA-based image fusion for skin burns assessment in polarization-sensitive OCT[J]. Biomed Opt Express, 2024, 15 (7): 4190−4205. doi: 10.1364/BOE.521399
[20] Sugita M, Pircher M, Zotter S, et al. Retinal nerve fiber bundle tracing and analysis in human eye by polarization sensitive OCT[J]. Biomed Opt Express, 2015, 6 (3): 1030−1054. doi: 10.1364/BOE.6.001030
[21] Parakkel R R, Wong D, Li C, et al. Retinal nerve fiber layer damage assessment in glaucomatous eyes using retinal retardance measured by polarization-sensitive optical coherence tomography[J]. Transl Vis Sci Technol, 2024, 13 (5): 9. doi: 10.1167/tvst.13.5.9
[22] Xu X, Luo Q, Wang J X, et al. Large-field objective lens for multi-wavelength microscopy at mesoscale and submicron resolution[J]. Opto-Electron Adv, 2024, 7: 230212. doi: 10.29026/oea.2024.230212
[23] Cense B, Chen T C, Park B H, et al. Thickness and birefringence of healthy retinal nerve fiber layer tissue measured with polarization-sensitive optical coherence tomography[J]. Invest Ophthalmol Vis Sci, 2004, 45 (8): 2606−2612. doi: 10.1167/iovs.03-1160
[24] Huang X R, Knighton R W. Microtubules contribute to the birefringence of the retinal nerve fiber layer[J]. Invest Ophthalmol Vis Sci, 2005, 46 (12): 4588−4593. doi: 10.1167/iovs.05-0532
[25] Al-Qaisi M K, Akkin T. Swept-source polarization-sensitive optical coherence tomography based on polarization-maintaining fiber[J]. Opt Express, 2010, 18 (4): 3392−3403. doi: 10.1364/OE.18.003392
[26] Tang P J, Xu J J, Wang R K. Imaging and visualization of the polarization state of the probing beam in polarization-sensitive optical coherence tomography[J]. Appl Phys Lett, 2018, 113 (23): 231101. doi: 10.1063/1.5050208.,
[27] Ortega-Quijano N, Marvdashti T, Ellerbee Bowden A K. Enhanced depolarization contrast in polarization-sensitive optical coherence tomography[J]. Opt Lett, 2016, 41 (10): 2350−2353. doi: 10.1364/OL.41.002350
[28] van der Meer F J, Faber D J, Aalders M C G, et al. Apoptosis- and necrosis-induced changes in light attenuation measured by optical coherence tomography[J]. Lasers Med Sci, 2010, 25 (2): 259−267. doi: 10.1007/s10103-009-0723-y
[29] Huang Y R, Zhang Z M, Tao W L, et al. Multiplexed stimulated emission depletion nanoscopy (mSTED) for 5-color live-cell long-term imaging of organelle interactome[J]. Opto-Electron Adv, 2024, 7: 240035. doi: 10.29026/oea.2024.240035
[30] Henry F P, Wang Y, Rodriguez C L R, et al. In vivo optical microscopy of peripheral nerve myelination with polarization sensitive-optical coherence tomography[J]. J Biomed Opt, 2015, 20 (4): 046002. doi: 10.1117/1.JBO.20.4.046002
[31] Jain N S, Jain S V, Wang X F, et al. Visualization of nerve fiber orientations in the human optic chiasm using photomicrographic image analysis[J]. Invest Ophthalmol Vis Sci, 2015, 56 (11): 6734−6739. doi: 10.1167/iovs.15-17443
[32] Li S X. Editorial: advances in CNS repair, regeneration, and neuroplasticity: from basic mechanisms to therapeutic strategies[J]. Front Cell Neurosci, 2022, 16: 898546. doi: 10.3389/fncel.2022.898546
[33] Grinblat G A, Khan R S, Dine K, et al. RGC neuroprotection following optic nerve trauma mediated by intranasal delivery of amnion cell secretome[J]. Invest Ophthalmol Vis Sci, 2018, 59 (6): 2470−2477. doi: 10.1167/iovs.18-24096
[34] Evanson N K, Guilhaume-Correa F, Herman J P, et al. Optic tract injury after closed head traumatic brain injury in mice: a model of indirect traumatic optic neuropathy[J]. PLoS One, 2018, 13 (5): e0197346. doi: 10.1371/journal.pone.0197346
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: