-
摘要:
近年来太赫兹光(0.1 THz~10 THz)因其良好的探测能力和非电离特性受到研究者们的关注。根据不同的检测方式和信号处理方法,可分为太赫兹成像技术和太赫兹光谱技术两大类。太赫兹技术在医学科学中发展迅速,其中生物大分子检测和组织成像令人印象深刻。水含量和结构差异是太赫兹成像技术的理论基础,据此可对生物组织进行检测识别。不同的生物组织具有不同的太赫兹特征谱,太赫兹光谱技术通过检测吸收系数、折射系数和反射系数来识别不同的生物分子、细胞或组织。实时、无标记的检测方式有望在临床实践中发挥重要作用,但仍需克服生物安全性不明等困难。综述介绍了太赫兹技术在医学科学中的应用及研究进展,同时探讨了太赫兹技术目前需要克服的难题和潜在的生物安全性问题。
Abstract:Terahertz radiation (0.1 THz~10 THz) has attracted extensive attention of researchers recently, because of its prominent detecting ability and its noninvasive and non-ionization properties. Terahertz technologies can be categorized into terahertz imaging and terahertz spectroscopy according to the manner of detection and signal processing. With the rapid development of terahertz technology, detection of macromolecule and imaging of tissues have achieved impressive progresses. Differences of water content and variations of structure or component are essential mechanisms of terahertz biomedical imaging, which was used in identifying different biomedical tissues. Terahertz spectroscopy is an edge technology for recognizing biomolecules, cells and tissues, based on their individual terahertz spectral fingerprints by assessing their absorbance, reflective and refractive index. Based on its properties, terahertz technology has great potential ability for clinical application, especially for real-time and label-free identification. However, this technology needs to overcome several difficulties, like biological safety. In this review, we introduce the applications of terahertz imaging and spectroscopy in medical science and medical research progress, and also discuss the difficulties of terahertz technology and potential biological safety.
-
Key words:
- terahertz imaging /
- terahertz spectroscopy /
- medicine
-

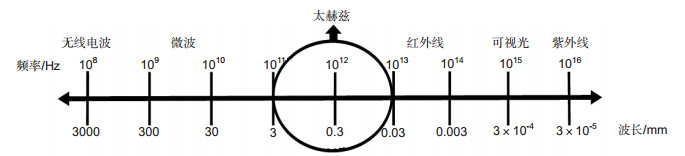
Overview: Terahertz radiation has attracted extensive attention of researchers recently, because of its prominent detecting ability and its noninvasive and non-ionization properties. The frequency band of terahertz radiation spans from 0.1 THz to 10 THz, which locates in the infrared regions and the microwave of electromagnetic spectrum. Terahertz technologies can be categorized into terahertz imaging and terahertz spectroscopy according to the manner of detection and signal processing. It is a typical interdisciplinary subject which combines electronics and photonics. With the rapid development of terahertz technology, it can be promisingly applied in the fields, such as biomedicine, quality control, security, national defense, environmental monitoring and astronomy. We introduce the current medical application of terahertz imaging and spectroscopy, ranging from vivo to vitro, from animals to human beings, from biomolecules to cells and tissues. Differences of water content and variations of structure or component are essential mechanisms of terahertz biomedical imaging. Setting pathological biopsy as gold standard for comparison, terahertz imaging has been widely used in identifying differences between normal tissues and abnormal tissues, including a variety of solid tumors, diabetic foots and flaps transplantation, etc. Compared with ultrasound, X-ray, computed tomography and magnetic resonance imaging, the comprehensive advantages of terahertz imaging are non-ionization and rapidness with acceptable sensitivity. Additionally, artificial contrast enhancement has been applied in terahertz imaging, such as gold nano-rods. In recent years, terahertz spectroscopy attracts a great deal of attention in probing and identifying various biomaterials. It is an edge technology for recognizing biomolecules, cells and tissues, based on their individual terahertz spectral fingerprints. Because the majority of low-frequency biomolecular motions lies in the terahertz spectrum, like rotation and vibration of the molecular skeleton. Time-domain spectroscopy and time resolved spectroscopy are common terahertz spectroscopy technologies, which analysis the differences of absorption coefficient or refraction index to probe and recognize a variety of biomolecules, cells or tissues. At present, terahertz medical application has achieved impressive progresses in discrimination, such as the detection of macromolecule and the imaging of tissues. Based on its properties, terahertz technology has great potential ability for clinical application, especially for real-time and label-free identification combined with or without pathology biopsy. However, this technology needs to overcome several difficulties, like biological safety, sample processing, detection performance and cost-efficient management. In this review, we introduce the application of terahertz imaging, spectroscopy in medical science and medical research progress, and also discuss the difficulties of terahertz technology and potential biological safety.
-

-
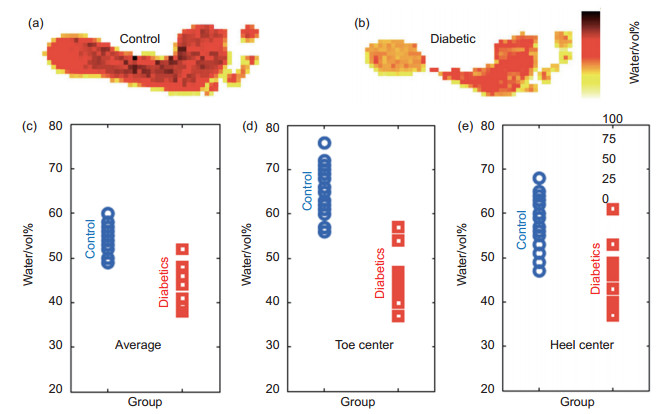
图 3 糖尿病足组与对照组的水含量的比较[22]。(a)对照组太赫兹成像图;(b)糖尿病足组太赫兹成像图;(c)足底水含量;(d)拇趾中心水含量;(e)脚后跟中心水含量
Figure 3. Comparison between diabetic group and control group[22]. (a) Terahertz image of a typical member of the control group; (b) Terahertz image of a typical member of the diabetic group; Volumetric fraction of water for control group members and diabetics (c) averaged over the foot sole, (d) at the center of the greater toe and (e) at the center of the heel
表 1 太赫兹成像技术的应用
Table 1. Applications of terahertz imaging
作者 年份 研究对象 标本处理方式 数量 太赫兹系统 分辨力 参考文献 Grootendorst 2017 乳腺恶性肿瘤 新鲜 46 太赫兹脉冲成像系统 1 mm [10] Fan 2017 皮肤黑色素瘤 新鲜 8 太赫兹脉冲成像系统 12 μm
(横向分辨力)[9] Bajwa 2017 小鼠皮瓣 烧伤 2 太赫兹反射成像系统 1 mm [18] Hernan-dez-Cardoso 2017 糖尿病足 足底 12 太赫兹反射成像系统 - [20] He 2016 猪的肌肉、脂肪 冰冻 6 太赫兹时域光谱及光栅扫描成像系统 476 μm [16] Jung 2011 淋巴结 石蜡包埋 5 太赫兹时域光谱及光栅扫描成像系统 2 mm [17] -
[1] Moulton P F. Spectroscopic and laser characteristics of Ti:Al2O3[J].Journal of the Optical Society of America B, 1986, 3(1): 125–133. doi: 10.1364/JOSAB.3.000125
[2] Bakopoulos P, Karanasiou I, Pleros N, et al. A tunable continuous wave (CW) and short-pulse optical source for THz brain imaging applications[J]. Measurement Science and Technology, 2009, 20(10): 104001. doi: 10.1088/0957-0233/20/10/104001
[3] Pawar A Y, Sonawane D D, Erande K B, et al. Terahertz technology and its applications[J]. Drug Invention Today, 2013, 5(2): 157–163. doi: 10.1016/j.dit.2013.03.009
[4] Shumyatsky P, Alfano R R. Terahertz sources[J]. Journal of Biomedical Optics, 2011, 16(3): 033001. doi: 10.1117/1.3554742
[5] Fan S T, He Y Z, Ung B S, et al. The growth of biomedical terahertz research[J]. Journal of Physics D: Applied Physics, 2014, 47(37): 374009. doi: 10.1088/0022-3727/47/37/374009
[6] 齐娜, 张卓勇, 相玉红.太赫兹技术在医学检测和诊断中的应用研究[J].光谱学与光谱分析, 2013, 33(8): 2064–2070. http://d.old.wanfangdata.com.cn/Periodical/gpxygpfx201308011
Qi N, Zhang Z Y, Xiang Y H. Application of terahertz technology in medical testing and diagnosis[J]. Spectroscopy and Spectral Analysis, 2013, 33(8): 2064–2070. http://d.old.wanfangdata.com.cn/Periodical/gpxygpfx201308011
[7] Yang X, Zhao X, Yang K, et al. Biomedical applications of terahertz spectroscopy and imaging[J]. Trends in Biotechnology, 2016, 34(10): 810–824. doi: 10.1016/j.tibtech.2016.04.008
[8] Hu B B, Nuss M C. Imaging with Terahertz Waves[J]. Optics Letters, 1995, 20(16): 1716–1718. doi: 10.1364/OL.20.001716
[9] Sy S, Huang S Y, Wang Y X J, et al. Terahertz spectroscopy of liver cirrhosis: investigating the origin of contrast[J]. Physics in Medicine & Biology, 2010, 55(24): 7587–7596.
[10] Grootendorst M R, Fitzgerald A J, de Koning S G B, et al. Use of a handheld terahertz pulsed imaging device to differentiate benign and malignant breast tissue[J]. Biomedical Optics Express, 2017, 8(6): 2932–2945. doi: 10.1364/BOE.8.002932
[11] Fan B, Neel V A, Yaroslavsky A N. Multimodal imaging for nonmelanoma skin cancer margin delineation[J]. Lasers in Surgery and Medicine, 2017, 49(3): 319–326. doi: 10.1002/lsm.22552
[12] Ahmed M, Rubio I T, Klaase J M, et al. Surgical treatment of nonpalpable primary invasive and in situ breast cancer[J]. Nature Reviews Clinical Oncology, 2015, 12(11): 645–663. doi: 10.1038/nrclinonc.2015.161
[13] Png G M, Choi J W, Ng B W H, et al. The impact of hydration changes in fresh bio-tissue on Thz spectroscopic measurements[J]. Physics in Medicine & Biology, 2008, 53(13): 3501–3517.
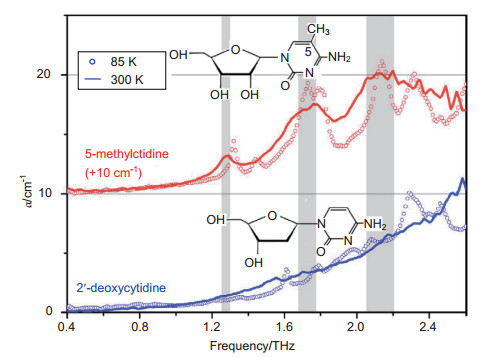
[14] Sun Y W, Fischer B M, Pickwell-MacPherson E. Effects of formalin fixing on the terahertz properties of biological tissues[J]. Journal of Biomedical Optics, 2009, 14(6): 064017. doi: 10.1117/1.3268439
[15] Fan S T, Ung B, Parrott E P J, et al. Gelatin embedding: a novel way to preserve biological samples for terahertz imaging and spectroscopy[J]. Physics in Medicine & Biology, 2015, 60(7): 2703–2713. http://adsabs.harvard.edu/abs/2015PMB....60.2703F
[16] Hoshina H, Hayashi A, Miyoshi N, et al. Terahertz pulsed imaging of frozen biological tissues[J]. Applied Physics Letters, 2009, 94(12): 123091. https://www.infona.pl/resource/bwmeta1.element.ieee-art-000005324920
[17] Sim Y C, Park J Y, Ahn K M, et al. Terahertz imaging of excised oral cancer at frozen temperature[J]. Biomedical Optics Express, 2013, 4(8): 1413–1421. doi: 10.1364/BOE.4.001413
[18] He Y Z, Ung B S, Parrott E P J, et al. Freeze-thaw hysteresis effects in terahertz imaging of biomedical tissues[J]. Biomedical Optics Express, 2016, 7(11): 4711–4717. doi: 10.1364/BOE.7.004711
[19] Jung E A, Lim M H, Moon K W, et al. Terahertz pulse imaging of micro-metastatic lymph nodes in early-stage cervical cancer patients[J]. Journal of the Optical Society of Korea, 2011, 15(2): 155–160. doi: 10.3807/JOSK.2011.15.2.155
[20] Bajwa N, Au J, Jarrahy R, et al. Non-invasive terahertz imaging of tissue water content for flap viability assessment[J]. Biomedical Optics Express, 2017, 8(1): 460–474. doi: 10.1364/BOE.8.000460
[21] Bajwa N, Sung S J, Ennis D B, et al. Terahertz imaging of cutaneous edema: correlation with magnetic resonance imaging in burn wounds[J]. IEEE Transactions on Biomedical Engineering, 2017, 64(11): 2682–2694. doi: 10.1109/TBME.2017.2658439
[22] Hernandez-Cardoso G G, Rojas-Landeros S C, Alfaro-Gomez M, et al. Terahertz imaging for early screening of diabetic foot syndrome: a proof of concept[J]. Scientific Reports, 2017, 7: 42124. doi: 10.1038/srep42124
[23] Kutteruf M R, Brown C M, Iwaki L K, et al. Terahertz spectroscopy of short-chain polypeptides[J]. Chemical Physics Letters, 2003, 375(3–4): 337–343. doi: 10.1016/S0009-2614(03)00856-X
[24] Kikuchi N, Tanno T, Watanabe M, et al. A membrane method for terahertz spectroscopy of amino acids[J]. Analytical Sciences, 2009, 25(3): 457–459. doi: 10.2116/analsci.25.457
[25] Ueno Y, Ajito K, Kukutsu N, et al. Quantitative analysis of amino acids in dietary supplements using terahertz time-domain spectroscopy[J]. Analytical Sciences, 2011, 27(4): 351. doi: 10.2116/analsci.27.351
[26] Lu S H, Zhang X, Zhang Z Y, et al. Quantitative measurements of binary amino acids mixtures in yellow foxtail millet by terahertz time domain spectroscopy[J]. Food Chemistry, 2016, 211: 494–501. doi: 10.1016/j.foodchem.2016.05.079
[27] Yamamoto K, Tominaga K, Sasakawa H, et al. Terahertz time-domain spectroscopy of amino acids and polypeptides[J]. Biophysical Journal, 2005, 89(3): L22–L24. https://www.sciencedirect.com/science/article/pii/S0006349505727910
[28] Xie L J, Yao Y, Ying Y B. The application of terahertz spectroscopy to protein detection: a review[J]. Applied Spectroscopy Reviews, 2014, 49(6): 448–461. doi: 10.1080/05704928.2013.847845
[29] Liu R, He M X, Su R X, et al. Insulin amyloid fibrillation studied by terahertz spectroscopy and other biophysical methods[J]. Biochemical and Biophysical Research Communications, 2010, 391(1): 862–867. doi: 10.1016/j.bbrc.2009.11.153
[30] Chen J Y, Knab J R, Ye S J, et al. Terahertz dielectric assay of solution phase protein binding[J]. Applied Physics Letters, 2007, 90(24): 243901. doi: 10.1063/1.2748852
[31] 滕学明, 田璐, 赵昆.太赫兹技术对营养品中蛋白质含量的研究[J].现代科学仪器, 2012(1): 91–94. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdkxyq201201023
Teng X M, Tian L, Zhao K. Investigation of protein content in nutriment by terahertz spectroscopy[J]. Modern Scientific Instruments, 2012(1): 91–94. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xdkxyq201201023
[32] Zou Y, Li J, Cui Y Y, et al. Terahertz spectroscopic diagnosis of myelin deficit brain in mice and rhesus monkey with chemometric techniques[J]. Scientific Reports, 2017, 7: 5176. doi: 10.1038/s41598-017-05554-z
[33] Yang J Q, Li S X, Zhao H W, et al. Molecular recognition and interaction between uracil and urea in solid-state studied by terahertz time-domain spectroscopy[J]. The Journal of Physical Chemistry A, 2014, 118(46): 10927–10933. doi: 10.1021/jp506045q
[34] Tang M J, Huang Q, Wei D S, et al. Terahertz spectroscopy of oligonucleotides in aqueous solutions[J]. Journal of Biomedical Optics, 2015, 20(9): 095009. doi: 10.1117/1.JBO.20.9.095009
[35] Cheon H, Yang H J, Lee S H, et al. Terahertz molecular resonance of cancer DNA[J]. Scientific Reports, 2016, 6: 37103. doi: 10.1038/srep37103
[36] Truong B C Q, Tuan H D, Fitzgerald A J, et al. A dielectric model of human breast tissue in terahertz regime[J]. IEEE Transactions on Biomedical Engineering, 2015, 62(2): 699–707. doi: 10.1109/TBME.2014.2364025
[37] Shi L Y, Shumyatsky P, Rodriguez-Contreras A, et al. Terahertz spectroscopy of brain tissue from a mouse model of Alzheimer's disease[J]. Journal of Biomedical Optics, 2016, 21(1): 015014. doi: 10.1117/1.JBO.21.1.015014
[38] Hou D B, Li X, Cai J H, et al. Terahertz spectroscopic investigation of human gastric normal and tumor tissues[J]. Physics in Medicine & Biology, 2014, 59(18): 5423–5440.
[39] Echchgadda I, Grundt J A, Tarango M, et al. Using a portable terahertz spectrometer to measure the optical properties of in vivo human skin[J]. Journal of Biomedical Optics, 2013, 18(12): 120503. doi: 10.1117/1.JBO.18.12.120503
[40] Reid C B, Reese G, Gibson A P, et al. Terahertz time-domain spectroscopy of human blood[J]. IEEE Journal of Biomedical and Health Informatics, 2013, 17(4): 774–778. doi: 10.1109/JBHI.2013.2255306
[41] Shiraga K, Ogawa Y, Suzuki T, et al. Characterization of dielectric responses of human cancer cells in the terahertz region[J]. Journal of Infrared, Millimeter, and Terahertz Waves, 2014, 35(5): 493–502. doi: 10.1007/s10762-014-0067-y
[42] Globus T, Dorofeeva T, Sizov I, et al. Sub-Thz vibrational spectroscopy of bacterial cells and molecular components[J]. American Journal of Biomedical Engineering, 2012, 2(4): 143–154. doi: 10.5923/j.ajbe.20120204.01
[43] Mazhorova A, Markov A, Ng A, et al. Label-free bacteria detection using evanescent mode of a suspended core terahertz fiber[J]. Optics Express, 2012, 20(5): 5344–5355. doi: 10.1364/OE.20.005344
[44] Wilmink G J, Grundt J E. Invited review article: current state of research on biological effects of terahertz radiation[J]. Journal of Infrared, Millimeter, and Terahertz Waves, 2011, 32(10): 1074–1122. doi: 10.1007/s10762-011-9794-5
[45] Alexandrov B S, Phipps M L, Alexandrov L B, et al. Specificity and heterogeneity of terahertz radiation effect on gene expression in mouse mesenchymal stem cells[J]. Scientific Reports, 2013, 3: 1184. doi: 10.1038/srep01184
[46] Wilmink G J, Rivest B D, Roth C C, et al. In vitro investigation of the biological effects associated with human dermal fibroblasts exposed to 2.52 THz radiation[J]. Lasers in Surgery and Medicine, 2011, 43(2): 152–163. doi: 10.1002/lsm.v43.2
[47] Borovkova M, Serebriakova M, Fedorov V, et al. Investigation of terahertz radiation influence on rat glial cells[J]. Biomedical Optics Express, 2017, 8(1): 273–280. doi: 10.1364/BOE.8.000273
[48] Williams R, Schofield A, Holder G, et al. The influence of high intensity terahertz radiation on mammalian cell adhesion, proliferation and differentiation[J]. Physics in Medicine & Biology, 2013, 58(2): 373–391.
[49] Oh S J, Kim S H, Jeong K, et al. Measurement depth enhancement in terahertz imaging of biological tissues[J]. Optics Express, 2013, 21(18): 21299–21305. doi: 10.1364/OE.21.021299
[50] Tuniz A, Kaltenecker K J, Fischer B M, et al. Metamaterial fibres for subdiffraction imaging and focusing at terahertz frequencies over optically long distances[J]. Nature Communications, 2013, 4: 2706.
[51] Ueno K, Nozawa S, Misawa H. Surface-enhanced terahertz spectroscopy using gold rod structures resonant with terahertz waves[J]. Optics Express, 2015, 23(22): 28584–28592. doi: 10.1364/OE.23.028584
[52] 尚丽平, 邓琥, 刘娟, 等.基于光导开关脉冲偏置电压的太赫兹时域光谱系统[J].光电工程, 2011, 38(3): 95–99. http://www.gdgc.ac.cn/CN/abstract/abstract973.shtml
Shang L P, Deng H, Liu J, et al. Terahertz time-domain spectrum system based on photoconductive semiconductor switch with offset voltage[J]. Opto-Electronic Engineering, 2011, 38(3): 95–99. http://www.gdgc.ac.cn/CN/abstract/abstract973.shtml
[53] Sun Y F, Sun J D, Zhou Y, et al. Room temperature GaN/AlGan self-mixing terahertz detector enhanced by resonant antennas[J]. Applied Physics Letters, 2011, 98(25): 252103. doi: 10.1063/1.3601489
[54] 孙云飞, 孙建东, 秦华, 等.基于硅透镜集成的高灵敏度室温太赫兹探测器[J].微纳电子技术, 2017, 54(11): 729–733. http://www.cqvip.com/QK/92139A/201302/44625638.html
Sun Y F, Sun J D, Qin H, et al. High sensitivity room temperature terahertz detector based on silicon lens integration[J]. Micronanoelectronic Technology, 2017, 54(11): 729–733. http://www.cqvip.com/QK/92139A/201302/44625638.html
[55] Nagel M, Bolivar P H, Brucherseifer M, et al. Integrated planar terahertz resonators for femtomolar sensitivity label-free detection of DNA hybridization[J]. Applied Optics, 2002, 41(10): 2074–2078. doi: 10.1364/AO.41.002074
[56] Park S J, Hong J T, Choi S J, et al. Detection of microorganisms using terahertz metamaterials[J]. Scientific Reports, 2014, 4: 4988. http://adsabs.harvard.edu/abs/2014NatSR...4E4988P
[57] 何明霞, 陈涛, 杨吉龙, 等.太赫兹成像技术在肿瘤诊断方面的应用[J].肿瘤, 2012, 32(12): 1039–1042. doi: 10.3781/j.issn.1000-7431.2012.12.016
He M X, Chen T, Yang J L, et al. The application of terahertz imaging in tumor diagnosis[J]. Tumor, 2012, 32(12): 1039–1042. doi: 10.3781/j.issn.1000-7431.2012.12.016
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: