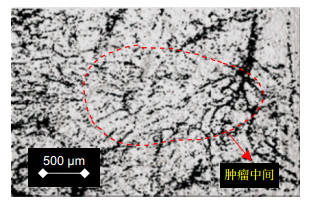
Imaging of skin structure and vessels in melanoma by swept source optical coherence tomography angiography
-
摘要
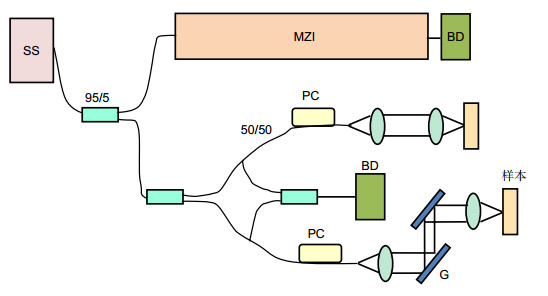
扫频源光学相干层析血管成像(SS-OCTA)是一种基于分频幅去相关血管造影法(SSADA)的新型血管成像技术,在肿瘤等疾病的早期诊断方面拥有较大前景。本文在5.12 mm×5.12 mm成像视场、标准图像最大信噪比34.3 dB的SS-OCTA成像平台,对黑色素瘤C57BL6小鼠进行皮肤结构和血管成像采集。结果表明在皮肤科疾病的早期诊断方面,利用SS-OCTA系统进行血管成像优于结构成像。

-
关键词:
- 扫频源光学相干层析血管成像 /
- 皮肤结构 /
- 肿瘤血管 /
- 黑色素瘤 /
- SS-OCTA
Abstract
Sweep source optical coherence tomographic angiography (SS-OCTA) is a kind of angiography technologies based on split spectrum amplitude deeorrelation angiography (SSADA). It has a great prospect in the early diagnosis of tumors and other diseases. In this paper, skin structure and angiography of melanoma C57BL6 mice were collected on the basis of the SS-OCTA imaging platform with an imaging field of 5.12 mm×5.12 mm and a standard image maximum signal-to-noise ratio of 34.3 dB. The results show that the SS-OCTA system is superior to the structural imaging in early diagnosis of dermatological diseases.
-
Overview

Overview: In recent years, optical coherence tomography (OCT) has developed rapidly and become a new imaging technology. OCT weakens coherent reflection and backscattering. Super heterodyne detection technique was used to improve the signal-to-noise ratio of biological tissue tomography. OCT has the advantages of non-invasive, high resolution, and high-speed imaging, and thus it is very suitable for biomedical applications. Scanning source optical coherence tomography (SS-OCTA) is a frequency-domain OCT technology and can support a high resolution in vivo angiography. As a new angiography technique, SS-OCTA still uses the Michelson interferometer's basic optical path and can achieve axial resolution of 15 microns by measuring the back scattering of light from low-coherent interference signals in tissue. Cross sectional images of 3D reconstruction of 3D images of biological tissues can be obtained, which are widely used in ophthalmology, dermatology imaging, tumor detection, and other fields. In addition to imaging biological tissue, SS-OCTA can also image surface blood vessels such as fundus and skin. SS-OCTA can observe the changes of retinal blood vessel morphology and blood flow in the choroid retina in the field of ophthalmology such as retinal angiography. Furthermore, it can also use pseudo-color to distinguish normal and abnormal vascular structures, blood flow signal detection and quantitative analysis, split different spectral images of the original full-spectrum image, reduce noise, improve signal-to-noise ratio, and then merge, so as to achieve retinal, choroidal vascular formation of any layer of significant cross-sectional imaging. Finally, we use laser speckle imaging and optical coherence tomography to noninvasive measurement of animal skin irritation and obtain dermal microvascular parameters. Angiography provides a possibility for the applications of SS-OCTA in the diagnosis of tumors, skin diseases, and other diseases. In fact, solid tumor growth is strongly dependent on the induced vascular network. Direct and indirect studies can support a strong evidence that tumor growth depends on blood vessels. Most tumors remain inactive until they become cancerous, and blood vessels no longer grow. Once entering the vascular phase, new blood vessels will grow rapidly to support tumor metabolism and play an important role in tumor proliferation. SS-OCTA can perform noninvasive imaging of biological tissues and blood vessels. This is of great significance for the early diagnosis of some tumors. Therefore, skin structure and angiography of melanoma C57BL6 mice were collected and compared with the SS-OCTA system. To observe the changes of the vascular development and biological tissue structure in the early stage of tumor growth, SS-OCTA is better at distinguishing vascular functional structures than the structural imaging.
-
-
-
参考文献
[1] Liang L, Jia Y L, Takusagawa H L, et al. Optical coherence tomography angiography of the peripapillary retina in Glaucoma[J]. Jama Ophthalmol, 2015, 133(9): 1045–1052. doi: 10.1001/jamaophthalmol.2015.2225
[2] 赵士勇, 俞信, 黄乃艳, 等. 大鼠耳部微血管光学相干层析成像研究[J].中国激光医学杂志, 2011, 20(3): 137–140, 202. http://d.old.wanfangdata.com.cn/Periodical/zgjgyx201103001
Zhao S Y, Yu X, Huang N Y, et al. Rat ear blood vessels imaging by optical coherence tomography[J]. Chinese Journal of Laser Medicine & Surgery, 2011, 20(3): 137–140, 202. http://d.old.wanfangdata.com.cn/Periodical/zgjgyx201103001
[3] 张玉梅, 杨琳, 戴培东, 等.光学相干层析成像技术在耳科学研究中的应用[J].中国眼耳鼻喉科杂志, 2018, 18(4): 285–288. http://d.old.wanfangdata.com.cn/Periodical/zgyebhkzz201804022
Zhang Y M, Yang L, Dai P D, et al. Application of optical coherence tomography in otology[J]. Chinese Journal of Ophthalmology and Otorhinolaryngology, 2018, 18(4): 285–288. http://d.old.wanfangdata.com.cn/Periodical/zgyebhkzz201804022
[4] 狄宇, 叶俊杰.光学相干层析扫描血管成像检查在眼科的应用[J].中华眼科杂志, 2017, 53(1): 65–72. doi: 10.3760/cma.j.issn.0412-4081.2017.01.014
Di Y, Ye J J. Application of optical coherence tomography angiography in ophthalmology[J]. Chinese Journal of Ophthalmology, 2017, 53(1): 65–72. doi: 10.3760/cma.j.issn.0412-4081.2017.01.014
[5] Gołębiewska J, Olechowski A, Wysocka-Mincewicz M, et al. Optical coherence tomography angiography vessel density in children with type 1 diabetes[J]. PLoS One, 2017, 12(10): e0186479. doi: 10.1371/journal.pone.0186479
[6] Zhang Q, Jonas J B, Wang Q, et al. Optical coherence tomography angiography vessel density changes after acute intraocular pressure elevation[J]. Scientific Reports, 2018, 8(1): 6024. doi: 10.1038/s41598-018-24520-x
[7] 孟庆刚.迈克尔逊干涉仪的应用[J].黑龙江科技信息, 2011(36): 62. doi: 10.3969/j.issn.1673-1328.2011.36.079
[8] Skalet A H, Li Y, Lu C D, et al. Optical coherence tomography angiography characteristics of iris melanocytic tumors[J]. Ophthalmology, 2017, 124(2): 197–204. doi: 10.1016/j.ophtha.2016.10.003
[9] Folkman J. Tumor angiogenesis: therapeutic implications[J]. The New England Journal of Medicine, 1971, 285(21): 1182–1186. doi: 10.1056/NEJM197111182852108
[10] Balch C M, Murad T M, Soong S J, et al. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods[J]. Annals of Surgery, 1978, 188(6): 732–742. doi: 10.1097/00000658-197812000-00004
[11] Fazlali H R, Karimi N, Soroushmehr S M R, et al. Vessel segmentation and catheter detection in X-ray angiograms using superpixels[J]. Medical & Biological Engineering & Computing, 2018, 56(9): 1515–1530. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1777ef55f3b391a0ebedfa5bda43e095
[12] Kato S, Kitagawa K, Ishida N, et al. Assessment of coronary artery disease using magnetic resonance coronary angiography: a national multicenter trial[J]. Journal of the American College of Cardiology, 2010, 56(12): 983–991. doi: 10.1016/j.jacc.2010.01.071
[13] 任杰, 王颖, 顾瑛.激光散斑成像和光学相干层析成像用于皮肤微血管无创检测的基础研究[J].中国激光医学杂志, 2012, 21(5): 309. http://d.old.wanfangdata.com.cn/Conference/8545671
Ren J, Wang Y, Gu Y. Basic research on noninvasive detection of skin microvasculature by laser speckle imaging and optical coherence yomography[J]. Chinese Journal of Laser Medicine & Surgery, 2012, 21(5): 309. http://d.old.wanfangdata.com.cn/Conference/8545671
[14] 喻超.
[15] 王倩, 魏文斌.分频幅去相干血管成像[J].国际眼科纵览, 2016, 40(2): 112–116. doi: 10.3760/cma.j.issn.1673-5803.2016.02.009
Wang Q, Wei W B. Optical coherence tomography angiography with split-spectrum amplitude decorrelation angiography[J]. International Review of Ophthalmology, 2016, 40(2): 112–116. doi: 10.3760/cma.j.issn.1673-5803.2016.02.009
[16] 高峰, 樊金宇, 孔文, 等.光学相干层析技术在血管流场检测方面的研究进展[J].中国激光, 2018, 45(2): 0207019. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgjg201802020
Gao F, Fan J Y, Kong W, et al. Research progress on optical coherence tomography in detecting vascular flow field[J]. Chinese Journal of Lasers, 2018, 45(2): 0207019. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgjg201802020
[17] Jia Y L, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography[J]. Optics Express, 2012, 20(4): 4710–4725. doi: 10.1364/OE.20.004710
[18] 罗斯特, 范应威, 常玮, 等. 扫频光学相干层析成像应用于判断黏液型胃癌边界区域[J].光学学报, 2018, 38(5): 0507001. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxxb201805032
Luo S T, Fan Y W, Chang W, et al. Boundary region of stomach mucinous carcinoma with swept source optical coherence tomography[J]. Acta Optica Sinica, 2018, 38(5): 0507001. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=gxxb201805032
[19] Cozzani E, Chinazzo C, Ghigliotti G, et al. Cutaneous angiosarcoma: the role of dermoscopy to reduce the risk of a delayed diagnosis[J]. International Journal of Dermatology, 2018, 57(8): 996–997. doi: 10.1111/ijd.13966
[20] Schoenenberger K, Colston B W, Maitland D J, et al. Mapping of birefringence and thermal damage in tissue by use of polarization-sensitive optical coherence tomography[J]. Applied Optics, 1998, 37(25): 6026–6036. doi: 10.1364/AO.37.006026
-
访问统计

 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: