-
摘要:
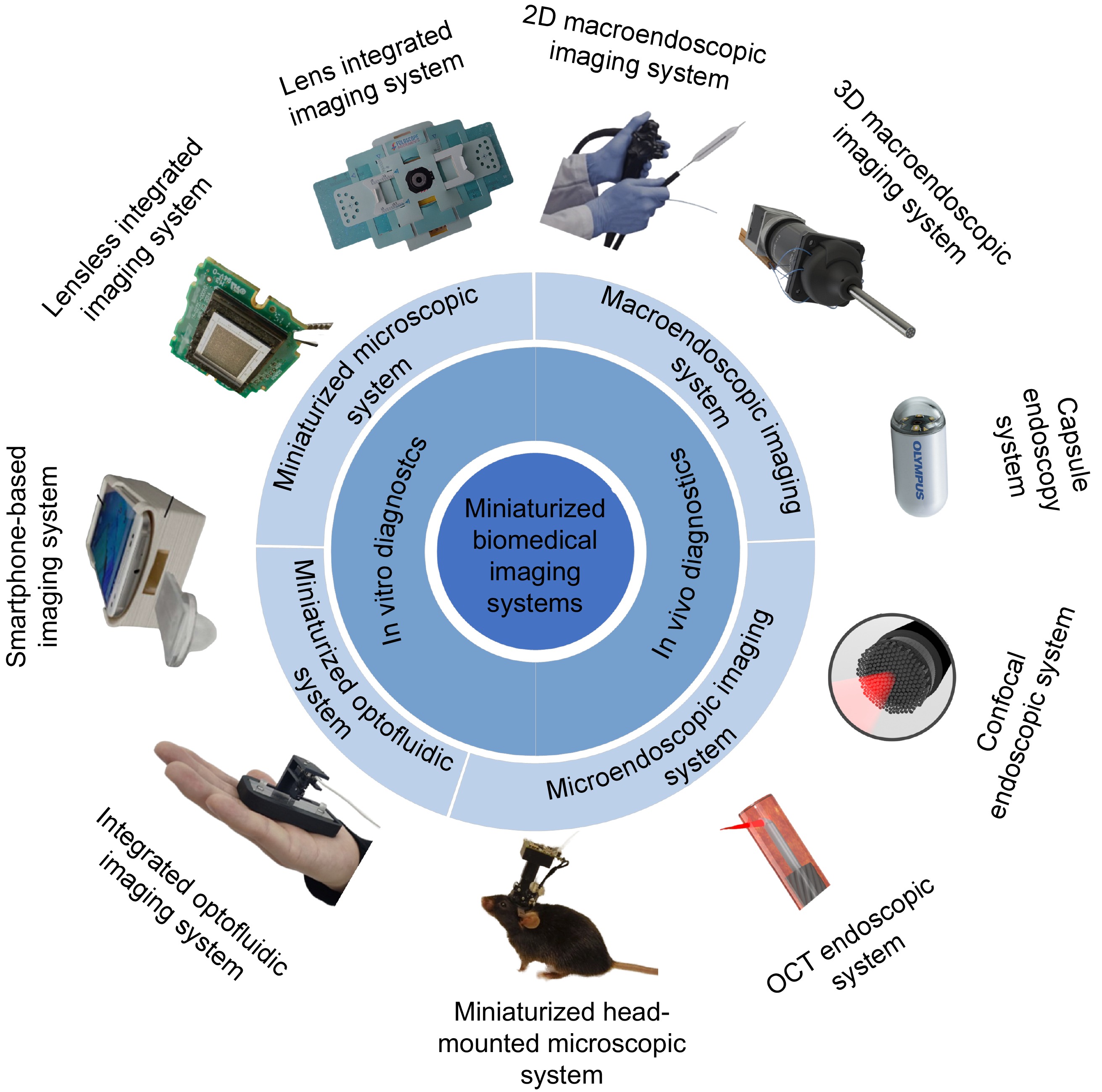
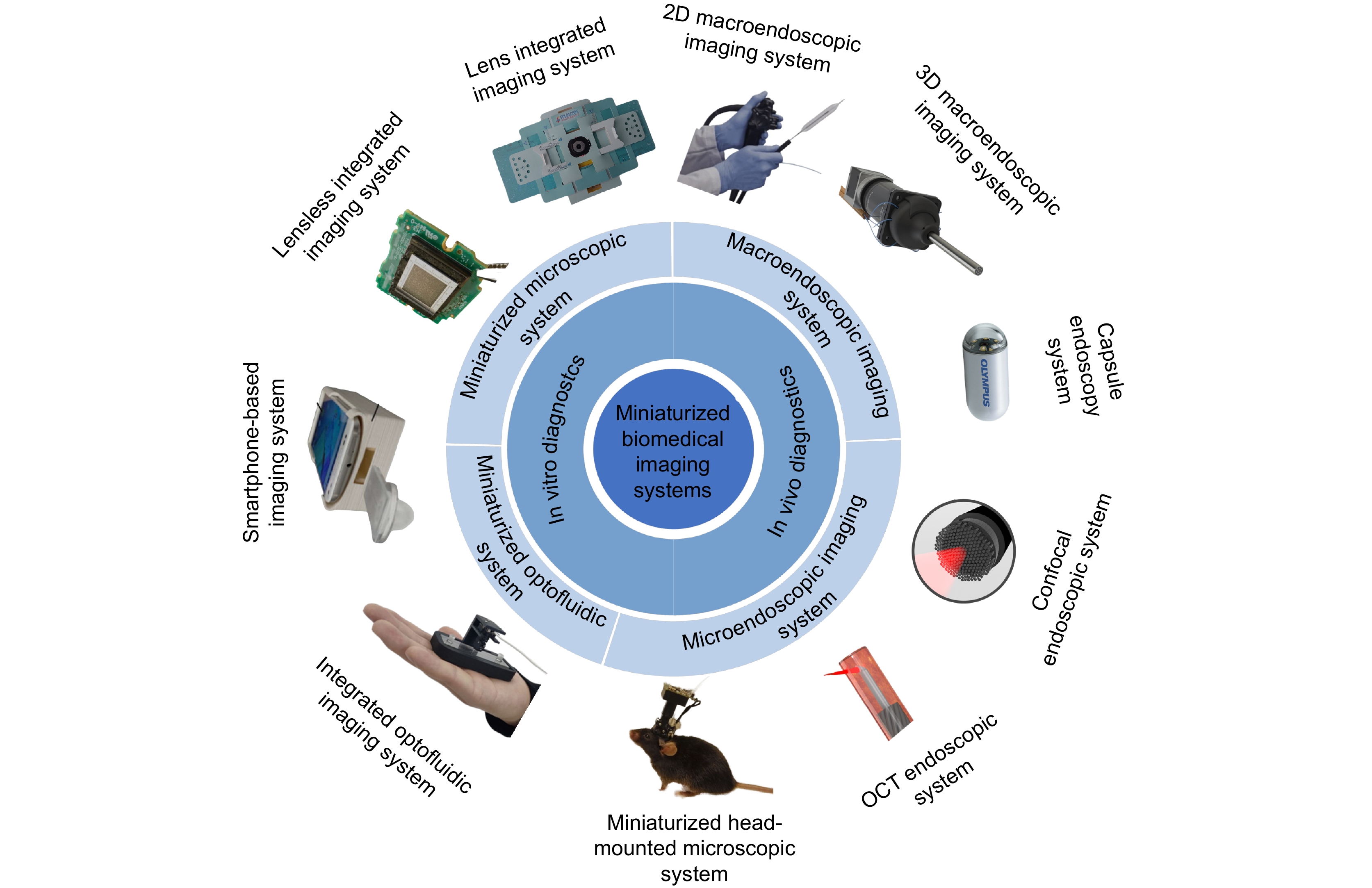
生物医学光学成像技术因其高分辨率和无辐射毒性的优势成为了连接基础科学研究与临床应用的桥梁,对推动医学进步和提高公共健康水平起到了重要作用。然而,生物医学光学成像系统往往需要将光、机、电、控元件整合为一体,导致设备笨重且昂贵,难以满足人们对即时诊断和体内微创高分辨率成像的需求。随着元件加工手段的进步和新式技术的引入,微型化生物医学光学成像系统的设计制造逐渐开始成为了新的研究热点之一。本文分别对用于体外和体内检测的微型化生物医学光学成像系统的基本原理、关键技术以及在临床中的具体应用进行了深入地综述和总结,最后展望了该领域的发展方向。
Abstract:Biomedical optical imaging techniques have become essential for bridging fundamental scientific research and clinical applications, owing to their high resolution and absence of radiation toxicity. These advancements are crucial for driving medical innovation and improving public health outcomes. However, the integration of optical, mechanical, electrical, and control components in biomedical optical imaging systems often leads to bulky and costly equipment. This complexity limits their capacity to fulfill the growing demand for point-of-care testing (POCT) and high-resolution, minimally invasive imaging. Recent advancements in component fabrication techniques and the emergence of new technologies have positioned the design and manufacture of miniaturized biomedical optical imaging systems as a significant area of research. This review comprehensively addresses the fundamental principles, key technologies, and specific clinical applications of these systems for both in vitro and in vivo detection. Lastly, we provide insights into potential future directions in this evolving field.
-
Key words:
- biomedical /
- optical imaging systems /
- miniaturization /
- endoscopic imaging systems
-
Overview: Recent advancements in biomedical optical imaging have profoundly reshaped medical diagnostics and research, offering high-resolution, non-invasive methods that effectively connect fundamental science with clinical applications. Despite their potential, traditional imaging systems are often hindered by issues of size, complexity, and cost, limiting their utility in real-time diagnostics and in vivo imaging. This manuscript provides an in-depth explanation of miniaturized biomedical optical imaging systems, emphasizing their design principles, technological innovations, and varied applications in both in vitro and in vivo contexts.This study highlights pivotal advancements in miniaturization techniques that have led to the development of compact imaging systems capable of delivering high-quality images while being user-friendly and cost-effective. We examine the fundamental principles underlying these systems, particularly the integration of optical components with micro-electromechanical systems (MEMS) and innovative imaging modalities such as two-photon and three-photon microscopy.In this investigation, we categorize miniaturized imaging systems into two primary modules: in vitro detection systems, which encompass microfluidic optical systems and miniaturized microscopy, and in vivo detection systems, including macro-endoscopic and microscopic endoscopic imaging. We present a series of experimental results that demonstrate the efficacy of these systems across various applications, such as sensitive cellular analysis, real-time monitoring of biological processes, and early disease detection. Importantly, our findings indicate that these miniaturized systems not only enhance imaging resolution but also significantly reduce the invasiveness of procedures, thereby improving patient comfort and clinical outcomes.The manuscript also discusses the integration of artificial intelligence (AI) and machine learning (ML) algorithms with miniaturized imaging systems, highlighting their potential to revolutionize image analysis and interpretation. By leveraging AI and ML, we foresee advancements in automated diagnostics, enabling rapid and accurate assessments in clinical settings.In conclusion, miniaturized biomedical optical imaging systems represent a paradigm shift in medical imaging, offering unprecedented opportunities for improving diagnostic capabilities and therapeutic monitoring. As research continues to advance in this field, these systems are poised to address critical global health challenges, ultimately leading to enhanced patient care and outcomes. Our study underscores the importance of continued innovation in imaging technologies, advocating for further exploration into their integration with emerging computational techniques to maximize their potential impact in healthcare.
-

-
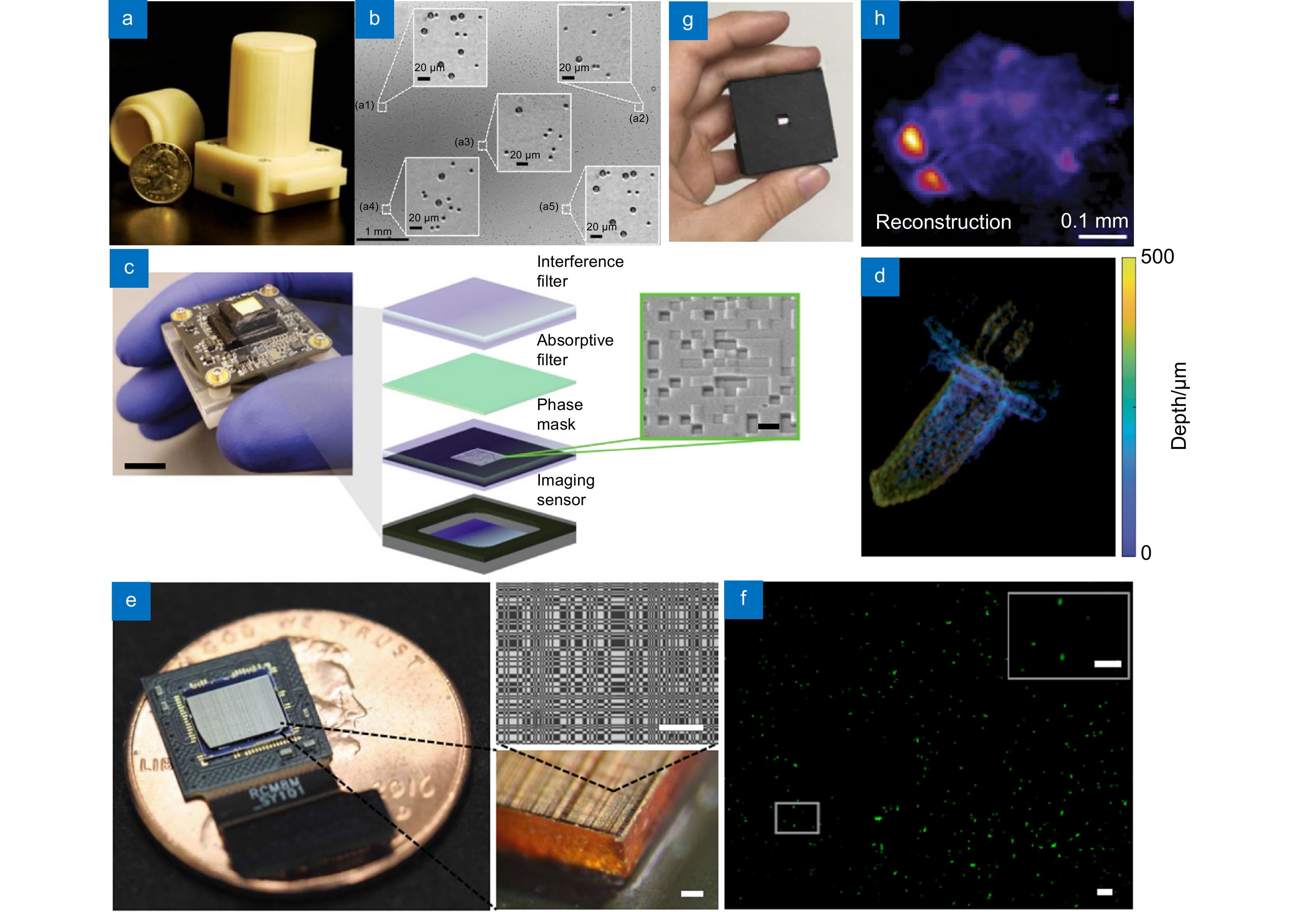
图 4 无透镜集成显微成像系统。(a) 基于非相干全息成像技术的紧凑型无透镜系统[45];(b) 由(a)设备拍摄的聚苯乙烯颗粒组成的样品的全视场DIC图像;(c) 高对比度无透镜系统[48];(d) 由(c)设备拍摄的固定水螅样本的重建的深度颜色编码图像;(e) 高分辨率3D荧光无透镜成像系统FlatScope[49]。标尺:100 μm;(f) 由(e)设备拍摄的荧光粒子重建图像。标尺:100 μm(插图中为50 μm);(g) 基于随机微透镜漫射器的高信噪比无透镜成像系统[51];(h) 由(g)设备拍摄的斑马鱼幼鱼样本。
Figure 4. Lensless integrated microscopic imaging system. (a) Compact lensless system based on incoherent holographic imaging system[45]; (b) A full-field DIC image of the sample composed of polystyrene particles, acquired using the (a) device; (c) High contrast lensless system[48]; (d) A reconstructed depth-coded image of the fixed Hydra sample, captured using the (c) device; (e) High-resolution 3D fluorescent lensless imaging system, FlatScope[49]. Scale bars of 100 μm; (f) A reconstructed image of fluorescent particles, acquired using the (e) device. Scale bars of 100 μm (50 μm in inset); (g) High SNR lensless imaging system based on a random microlens diffuser[51]; (h) Zebrafish larval sample imaged using the (g) device.
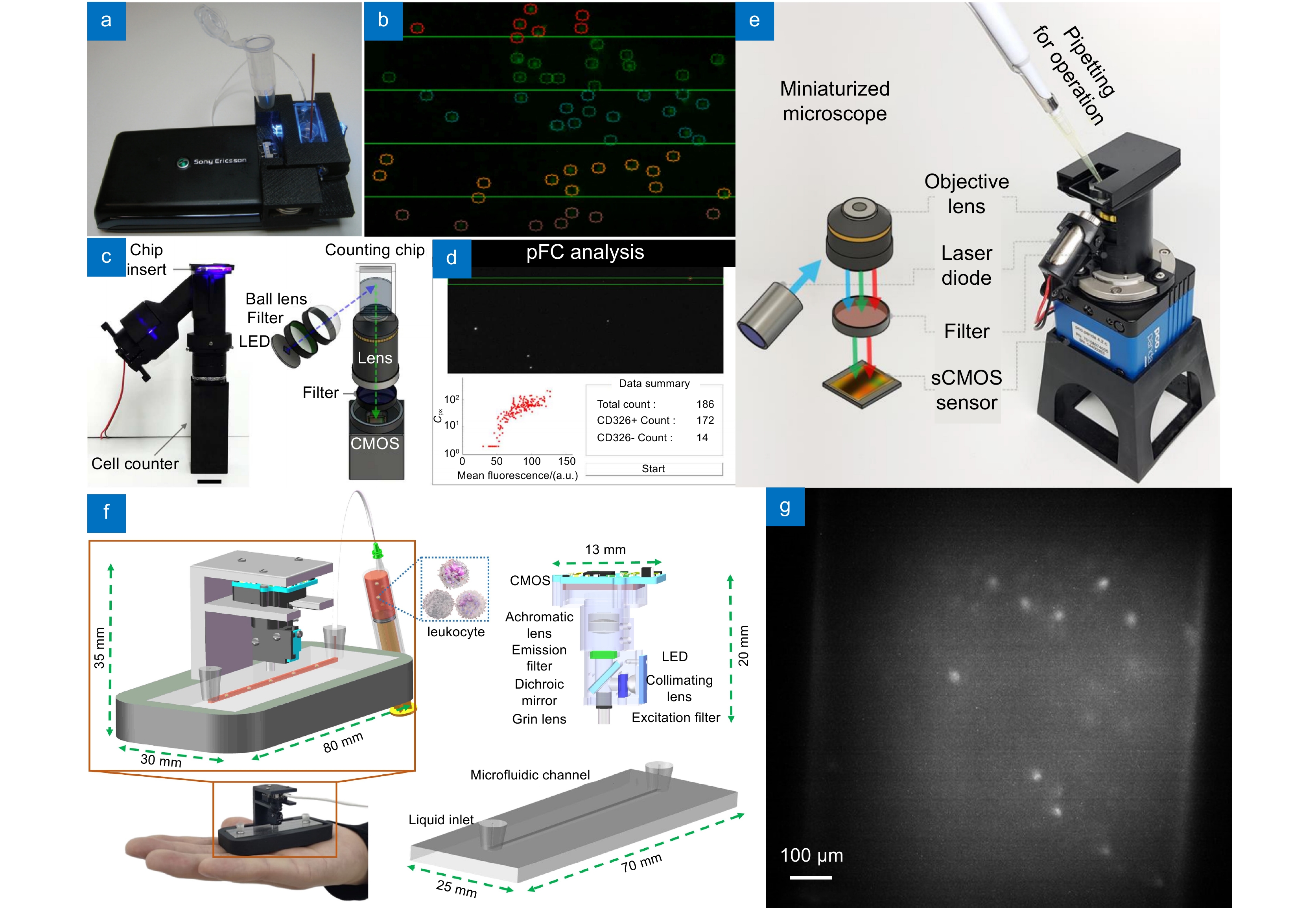
图 6 微型化光流控流式细胞仪。(a) 基于手机平台的流式细胞仪[56]; (b) 图(a)系统的细胞计数界面; (c) 无泵式流式细胞仪[57]; (d) 图(c)系统的细胞计数界面; (e) 集成式微型流式细胞仪[58]; (f) 智能掌上光流控血细胞计数仪[35]; (g) 图(f)系统流道内白细胞的成像效果
Figure 6. Miniaturized optofluidic flow cytometer. (a) Miniaturized optofluidic flow cytometer based on a mobile platform[56]; (b) The cell counting interface of the system in Figure (a); (c) Pumpless flow cytometer[57]; (d) The cell counting interface of the system in Figure (c); (e) Integrated miniaturized flow cytometer[58]; (f) Smart palm-size optofluidic hematology analyzer[35]; (g) Figure (f) Imaging effect of white blood cells in the system channel
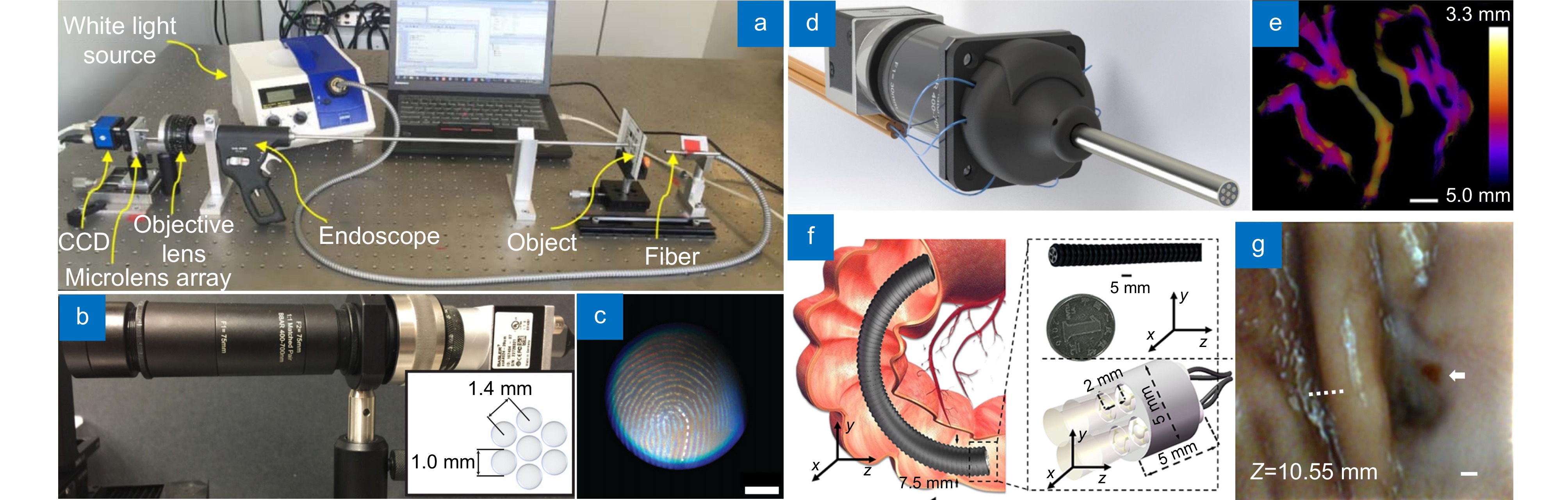
图 9 基于光场技术的3D内窥镜。(a) 基于微透镜阵列的刚性光场内窥镜[86]; (b) 基于Grin lens阵列的光场内窥镜验证机[76]; (c) 基于图(b)设备拍摄的指纹样本,比例尺:2.0 mm; (d) 基于Grin lens阵列的光场内窥镜原型机(GLAM)[36]; (e) 基于图(d)系统拍摄的三维样品; (f) 高柔性光场内镜[11]; (g) 基于图(f)系统拍摄的兔子胃部深度图,比例尺:500 μm
Figure 9. 3D endoscopes based on light-field method. (a) Rigid light-field endoscope based on microlens[86]; (b) Verification machine of light-field endoscope based on Grin lens array[76]; (c) Fingerprint sample taken by the device based on Figure (b), scale bar of 2.0 mm; (d) Prototype of light-field endoscope based on Grin lens array[36]; (e) 3D samples taken based on Figure (d) system; (f) Highly flexible light-field endoscope[11]; (g) Rabbit stomach depth map based on system of Figure (f), scale bar of 500 μm
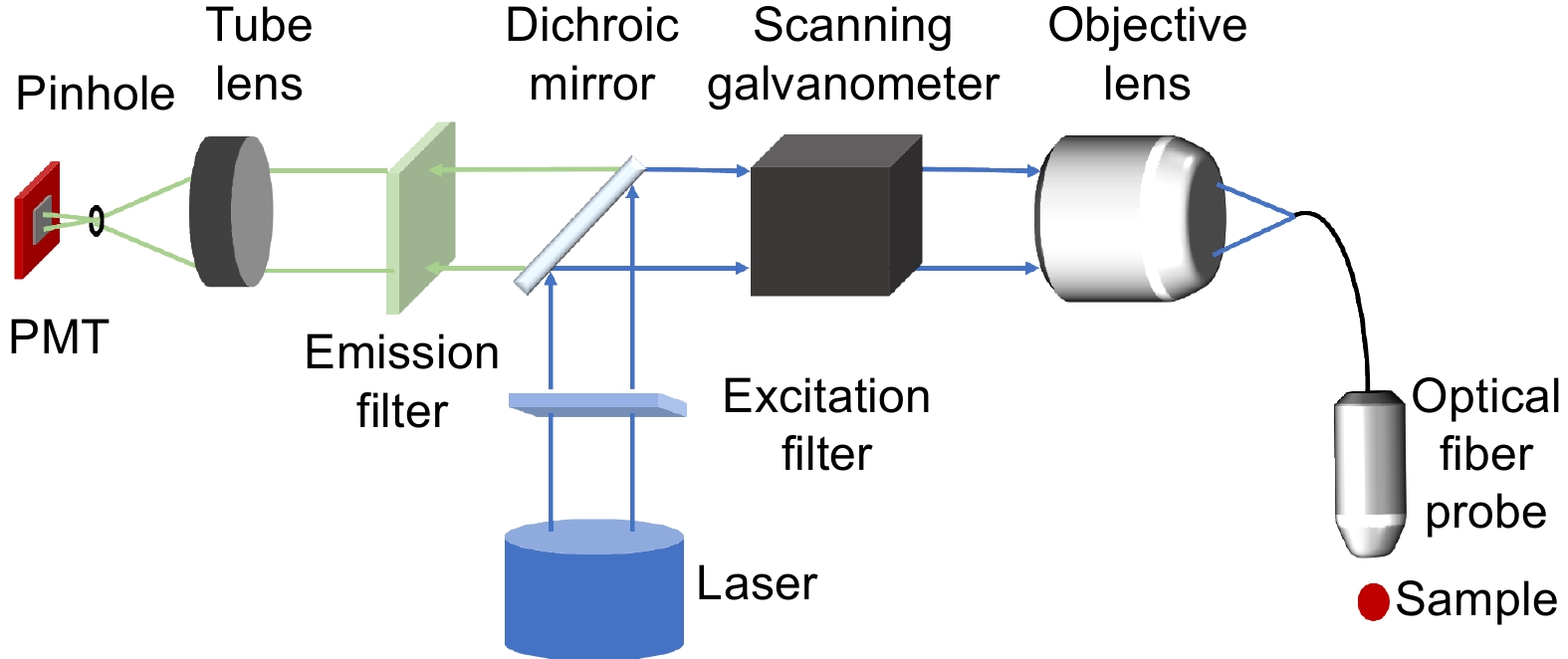
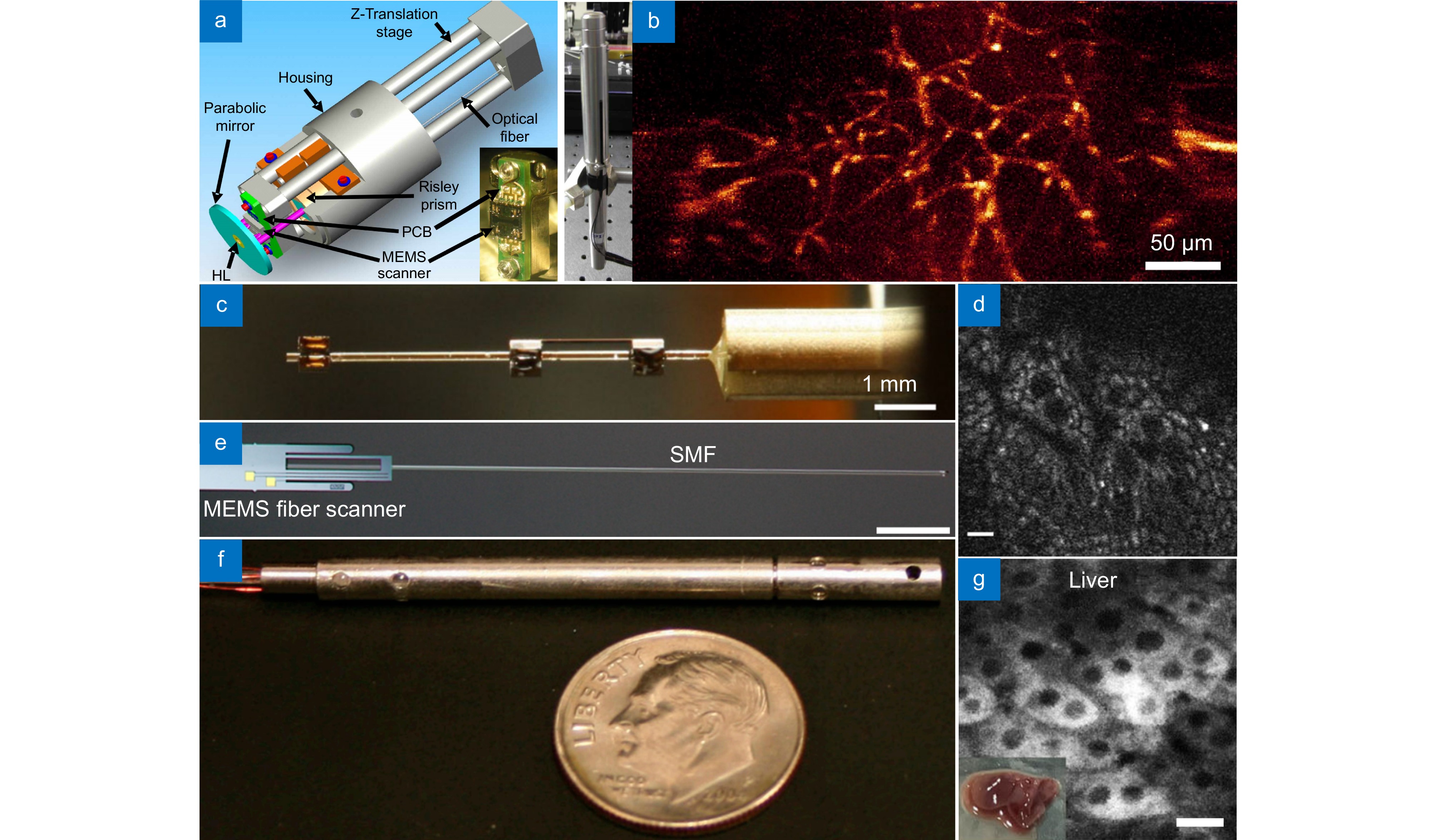
图 12 共聚焦内窥成像系统。(a) 手持双轴刚性共聚焦内窥镜[94]; (b) 通过图(a)设备拍摄的小鼠离体组织图像; (c) 基于光纤谐波仪的手持式共聚焦内窥成像系统[96]; (d) 由图(c)设备拍摄的小鼠离体结肠图片,比例尺:10 μm; (e) 基于电热MEMS激光扫描内窥成像系统[99],比例尺:2 mm; (f) 基于双光子和二次谐波成像的内窥成像系统[102]; (g) 由图(f)设备拍摄的小鼠离体肝细胞图片,比例尺:20 μm
Figure 12. Confocal endoscopic imaging system. (a) Handheld dual-axes confocal endoscope[94]; (b) Image of a mouse tissue taken in vitro by the device in Figure (a); (c) Handheld endomicroscope using a fiber-optic harmonograph[96]; (d) Ex vivo image of isolated mouse colon cells taken by the device in Figure (c), scale bar of 10 μm; (e) Electrothermal MEMS fiber scanner for optical endomicroscopy[99], scale bar of 2 mm; (f) Endoscopic imaging system based on two-photon and second harmonic imaging[102]; (g) Image of isolated mouse liver cells taken by the device in Figure (f), scale bar of 20 μm
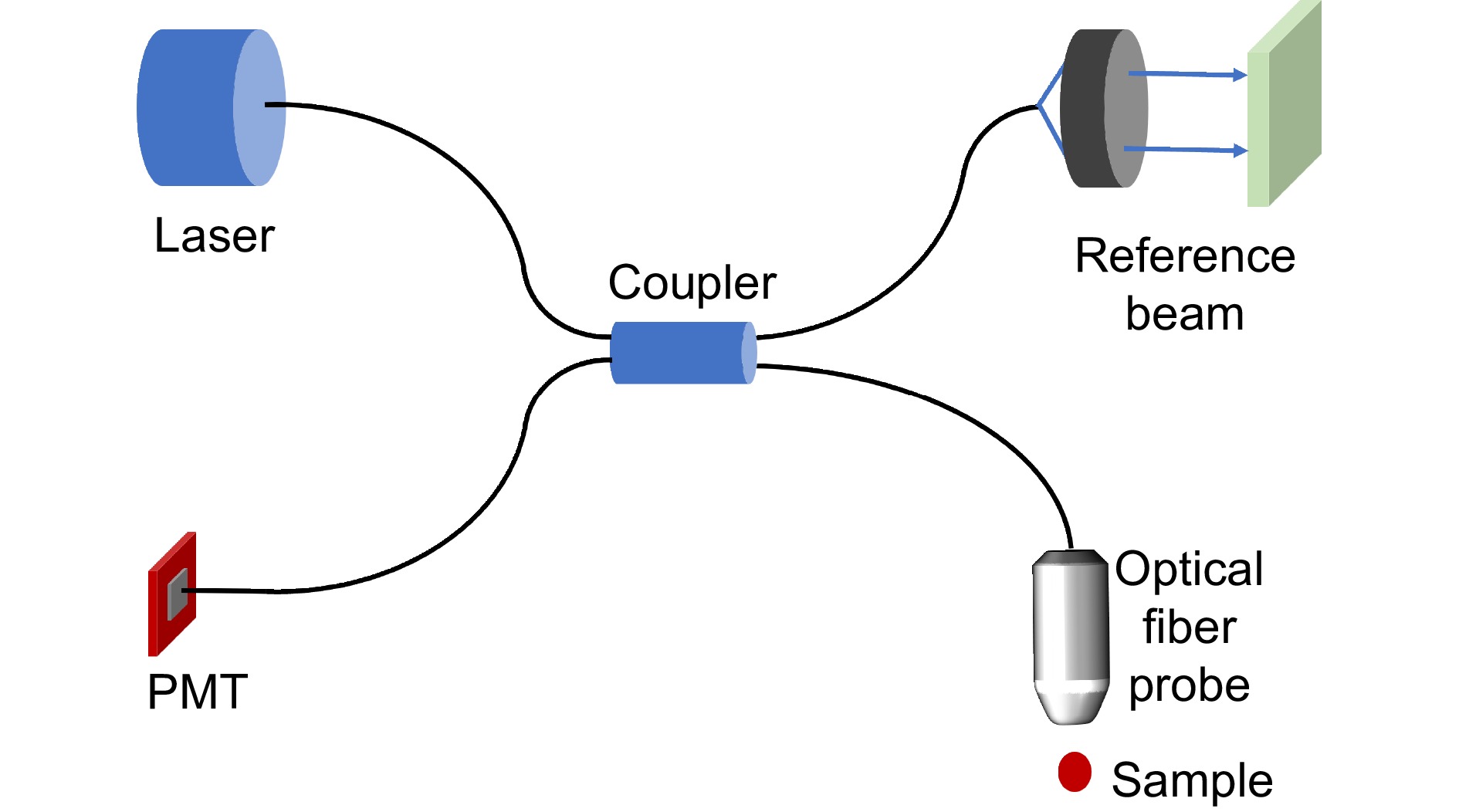
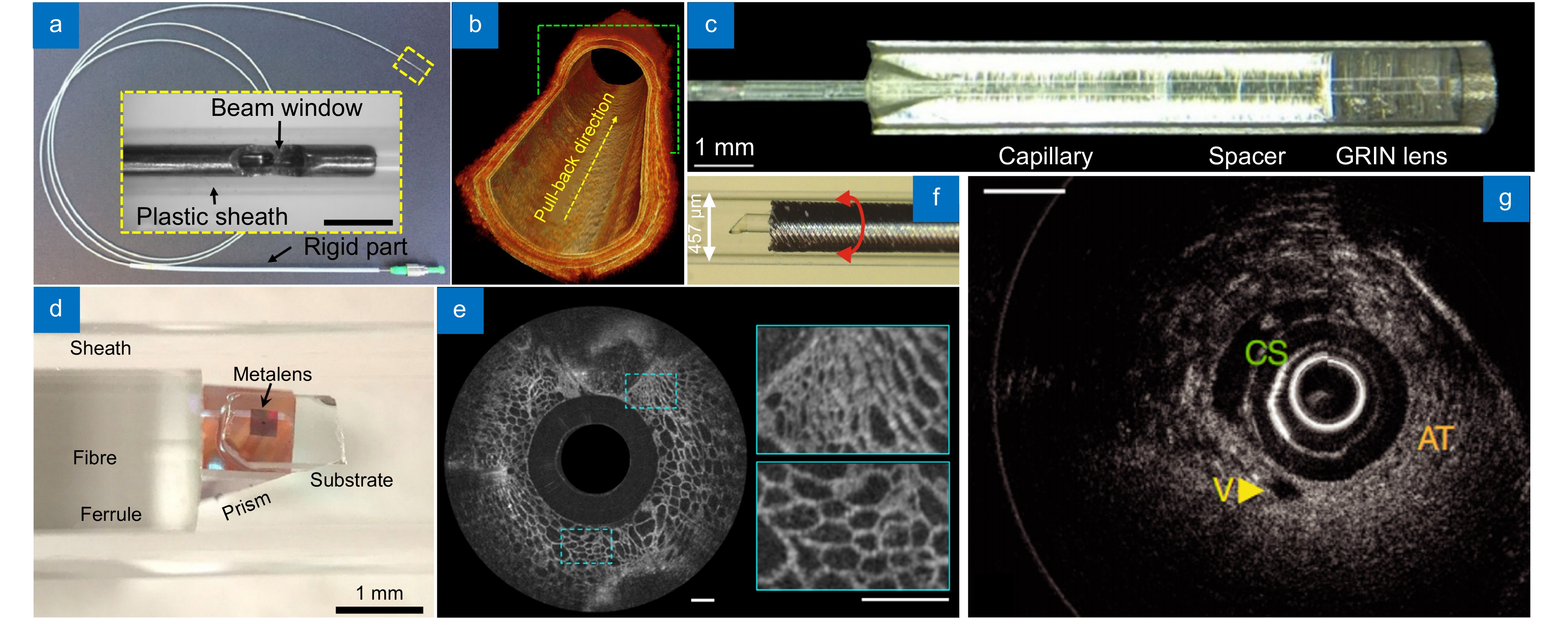
图 14 OCT内窥系统。(a) 高消色差OCT微探针[123]; (b) 由图(a)中的设备拍摄的4 mm长的大鼠食管; (c) 带有相位滤波器的光学探针[124]; (d) 纳米光学OCT内窥镜[125]; (e) 由图(d)中的设备对猪气道的离体成像,比例尺:500 μm; (f) 超薄单片三维打印OCT内窥镜[32]; (g) 由图(f)中的设备对健康小鼠动脉血管的成像
Figure 14. OCT endoscopic system. (a) Super-achromatic OCT microprobe[123]; (b) 4 mm-long rat esophagi imaged by the device shown in Figure (a); (c) Optical probe with a phase filter[124]; (d) Nano-optic OCT endoscope[125]; (e) Ex vivo imaging of the pig trachea using the device shown in Figure (d), scale bar of 500 μm; (f) Ultrathin monolithic 3D printed OCT endoscope[32]; (g) Imaging of the arterial vasculature in healthy mice using the device shown in Figure (f)
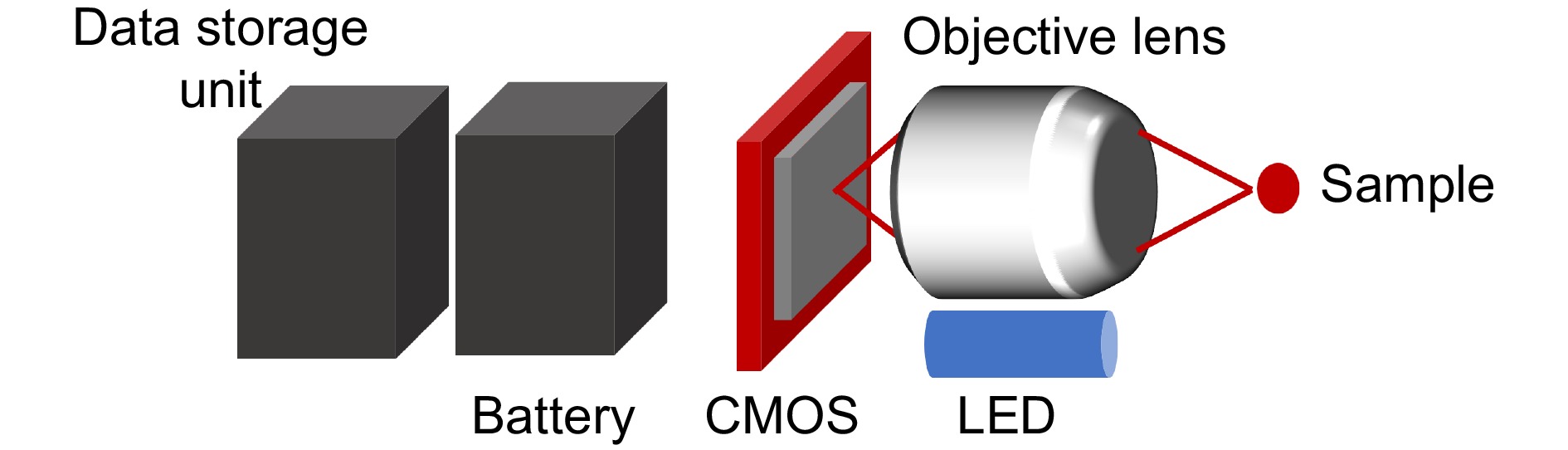
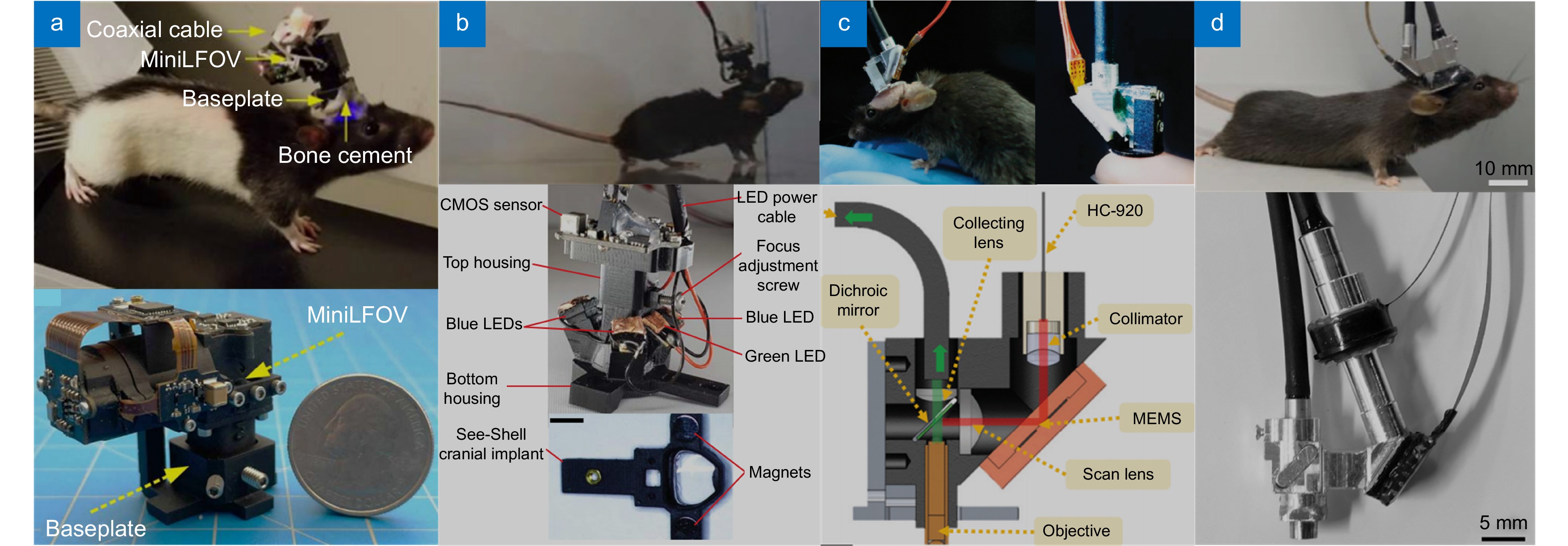
图 16 微型化显微成像系统。(a) 大视场微型化显微镜(MiniLFOV)[133]; (b) 微型化全脑中微镜(mini-mScope)[134];(c) 微型化双光子显微镜(FHIRM-TPM)[142]; (d) 微型化三光子显微镜(m3PM)[144]
Figure 16. Miniaturized microscopic imaging system. (a) Large-field-of-view miniscope(MiniLFOV)[133]; (b) Miniaturized microscope for whole-cortex mesoscale imaging(mini-mScope)[134]; (c) Miniaturized two photon microscope(FHIRM-TPM)[142]; (d) Miniaturized three photon microscope(m3PM)[144]
-
[1] Kellerer T, Sailer B, Byers P, et al. Two-photon microscopy of acoustofluidic trapping for highly sensitive cell analysis[J]. Lab Chip, 2024, 24(14): 3456−3469. doi: 10.1039/D4LC00144C
[2] Lim J, Chin V, Fairfax K, et al. Transitioning single-cell genomics into the clinic[J]. Nat Rev Genet, 2023, 24(8): 573−584. doi: 10.1038/s41576-023-00613-w
[3] Shi J P, Tian Z X, Lai J S, et al. Plant pan-genomics and its applications[J]. Mol Plant, 2023, 16(1): 168−186. doi: 10.1016/j.molp.2022.12.009
[4] Theissinger K, Fernandes C, Formenti G, et al. How genomics can help biodiversity conservation[J]. Trends Genet, 2023, 39(7): 545−559. doi: 10.1016/j.tig.2023.01.005
[5] Naba A. Ten years of extracellular matrix proteomics: accomplishments, challenges, and future perspectives[J]. Mol Cell Proteomics, 2023, 22(4): 100528. doi: 10.1016/j.mcpro.2023.100528
[6] Yu F C, Teo G C, Kong A T, et al. Analysis of DIA proteomics data using MSFragger-DIA and FragPipe computational platform[J]. Nat Commun, 2023, 14(1): 4154. doi: 10.1038/s41467-023-39869-5
[7] Emmerich C H, Gamboa L M, Hofmann M C J, et al. Improving target assessment in biomedical research: the GOT-IT recommendations[J]. Nat Rev Drug Discov, 2021, 20(1): 64−81. doi: 10.1038/s41573-020-0087-3
[8] Pan Y C, Hu X Y, Guo D S. Biomedical applications of calixarenes: state of the art and perspectives[J]. Angew Chem Int Ed, 2021, 60(6): 2768−2794. doi: 10.1002/anie.201916380
[9] Clasky A J, Watchorn J D, Chen P Z, et al. From prevention to diagnosis and treatment: biomedical applications of metal nanoparticle-hydrogel composites[J]. Acta Biomater, 2021, 122: 1−25. doi: 10.1016/j.actbio.2020.12.030
[10] Hamedi H, Moradi S, Hudson S M, et al. Chitosan based bioadhesives for biomedical applications: a review[J]. Carbohydr Polym, 2022, 282: 119100. doi: 10.1016/j.carbpol.2022.119100
[11] Su D E, Gao W D, Li H Y, et al. Highly flexible and compact volumetric endoscope by integrating multiple micro-imaging devices[J]. Opt Lett, 2023, 48(24): 6416−6419. doi: 10.1364/OL.506261
[12] Su D E, Wang X W, Shang G Y, et al. Amplitude-phase modulation metasurface hologram with inverse angular spectrum diffraction theory[J]. J Phys D Appl Phys, 2022, 55(23): 235102. doi: 10.1088/1361-6463/ac5699
[13] 苏德尔, 李浩宇, 高伟达, 等. 用于管道检测机器人的微型化成像系统(特邀)[J]. 激光与光电子学进展, 2024, 61(2): 0211013. doi: 10.3788/LOP232771
Su D E, Li H Y, Gao W D, et al. Miniaturized imaging system designed for pipeline detection robots (invited)[J]. Laser Optoelectron Prog, 2024, 61(2): 0211013. doi: 10.3788/LOP232771
[14] Wang X W, Wang H, Wang J L, et al. Single-shot isotropic differential interference contrast microscopy[J]. Nat Commun, 2023, 14(1): 2063. doi: 10.1038/s41467-023-37606-6
[15] Zhao W S, Huang X S, Yang J Y, et al. Quantitatively mapping local quality of super-resolution microscopy by rolling Fourier ring correlation[J]. Light Sci Appl, 2023, 12(1): 298. doi: 10.1038/s41377-023-01321-0
[16] Zhao W S, Zhao S Q, Han Z Q, et al. Enhanced detection of fluorescence fluctuations for high-throughput super-resolution imaging[J]. Nat Photonics, 2023, 17(9): 806−813. doi: 10.1038/s41566-023-01234-9
[17] Zhao W S, Zhao S Q, Li L J, et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy[J]. Nat Biotechnol, 2022, 40(4): 606−617. doi: 10.1038/s41587-021-01092-2
[18] Stachelek P, MacKenzie L, Parker D, et al. Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions[J]. Nat Commun, 2022, 13(1): 553. doi: 10.1038/s41467-022-28220-z
[19] Peeling R W, Mabey D. Point-of-care tests for diagnosing infections in the developing world[J]. Clin Microbiol Infect, 2010, 16(8): 1062−1069. doi: 10.1111/j.1469-0691.2010.03279.x
[20] Koetzier L R, Mastrodicasa D, Szczykutowicz T P, et al. Deep learning image reconstruction for CT: technical principles and clinical prospects[J]. Radiology, 2023, 306(3): 221257. doi: 10.1148/RADIOL.221257
[21] Güngör A, Dar S U H, Öztürk Ş, et al. Adaptive diffusion priors for accelerated MRI reconstruction[J]. Med Image Anal, 2023, 88: 102872. doi: 10.1016/j.media.2023.102872
[22] Zhu H Y, Isikman S O, Mudanyali O, et al. Optical imaging techniques for point-of-care diagnostics[J]. Lab Chip, 2013, 13(1): 51−67. doi: 10.1039/C2LC40864C
[23] Holzner G, Du Y, Cao X B, et al. An optofluidic system with integrated microlens arrays for parallel imaging flow cytometry[J]. Lab Chip, 2018, 18(23): 3631−3637. doi: 10.1039/C8LC00593A
[24] Boominathan V, Robinson J T, Waller L, et al. Recent advances in lensless imaging[J]. Optica, 2022, 9(1): 1−16. doi: 10.1364/OPTICA.431361
[25] Paiè P, Bragheri F, Claude T, et al. Optofluidic light modulator integrated in lab-on-a-chip[J]. Opt Express, 2017, 25(7): 7313−7323. doi: 10.1364/OE.25.007313
[26] Mwachiro M M, Burgert S L, Lando J, et al. Esophageal squamous dysplasia is common in asymptomatic kenyans: a prospective, community-based, cross-sectional study[J]. Am J Gastroenterol, 2016, 111(4): 500−507. doi: 10.1038/ajg.2016.26
[27] Asif M S, Ayremlou A, Sankaranarayanan A, et al. FlatCam: thin, lensless cameras using coded aperture and computation[J]. IEEE Trans Comput Imaging, 2017, 3(3): 384−397. doi: 10.1109/TCI.2016.2593662
[28] Ciuti G, Caliò R, Camboni D, et al. Frontiers of robotic endoscopic capsules: a review[J]. J Micro-Bio Robot, 2016, 11(1): 1−18. doi: 10.1007/s12213-016-0087-x
[29] Cybulski J S, Clements J, Prakash M. Foldscope: Origami-Based Paper Microscope[J]. PLoS ONE, 2014, 9(6): e98781. doi: 10.1371/journal.pone.0098781
[30] Gordon G S D, Joseph J, Alcolea M P, et al. Quantitative phase and polarization imaging through an optical fiber applied to detection of early esophageal tumorigenesis[J]. J Biomed Opt, 2019, 24(12): 126004. doi: 10.1117/1.JBO.24.12.126004
[31] Kanakasabapathy M K, Sadasivam M, Singh A, et al. An automated smartphone-based diagnostic assay for point-of-care semen analysis[J]. Sci Transl Med, 2017, 9(382): eaai7863. doi: 10.1126/scitranslmed.aai7863
[32] Li J W, Thiele S, Quirk B C, et al. Ultrathin monolithic 3D printed optical coherence tomography endoscopy for preclinical and clinical use[J]. Light Sci Appl, 2020, 9(1): 124. doi: 10.1038/s41377-020-00365-w
[33] Quang T, Schwarz R A, Dawsey S M, et al. A tablet-interfaced high-resolution microendoscope with automated image interpretation for real-time evaluation of esophageal squamous cell neoplasia[J]. Gastrointest Endosc, 2016, 84(5): 834−841. doi: 10.1016/j.gie.2016.03.1472
[34] Skocek O, Nöbauer T, Weilguny L, et al. High-speed volumetric imaging of neuronal activity in freely moving rodents[J]. Nat Methods, 2018, 15(6): 429−432. doi: 10.1038/s41592-018-0008-0
[35] Su D E, Li X Y, Gao W D, et al. Smart palm-size optofluidic hematology analyzer for automated imaging-based leukocyte concentration detection[J]. Opto Electron Sci, 2023, 2(12): 230018. doi: 10.29026/oes.2023.230018
[36] Urner T M, Inman A, Lapid B, et al. Three-dimensional light-field microendoscopy with a GRIN lens array[J]. Biomed Opt Express, 2022, 13(2): 590−607. doi: 10.1364/BOE.447578
[37] Wallace M B, Fockens P. Probe-based confocal laser endomicroscopy[J]. Gastroenterology, 2009, 136(5): 1509−1513. doi: 10.1053/j.gastro.2009.03.034
[38] Salido J, Bueno G, Ruiz-Santaquiteria J, et al. A review on low-cost microscopes for open science[J]. Microsc Res Tech, 2022, 85(10): 3270−3283. doi: 10.1002/jemt.24200
[39] Zhang Y S, Ribas J, Nadhman A, et al. A cost-effective fluorescence mini-microscope for biomedical applications[J]. Lab Chip, 2015, 15(18): 3661−3669. doi: 10.1039/C5LC00666J
[40] Zhu H Y, Yaglidere O, Su T W, et al. Cost-effective and compact wide-field fluorescent imaging on a cell-phone[J]. Lab Chip, 2011, 11(2): 315−322. doi: 10.1039/C0LC00358A
[41] Orth A, Wilson E R, Thompson J G, et al. A dual-mode mobile phone microscope using the onboard camera flash and ambient light[J]. Sci Rep, 2018, 8(1): 3298. doi: 10.1038/s41598-018-21543-2
[42] Rabha D, Sarmah A, Nath P. Design of a 3D printed smartphone microscopic system with enhanced imaging ability for biomedical applications[J]. J Microsc, 2019, 276(1): 13−20. doi: 10.1111/jmi.12829
[43] Cesaretti M, Gal J, Bouveyron C, et al. Accurate assessment of nonalcoholic fatty liver disease lesions in liver allograft biopsies by a smartphone platform: a proof of concept[J]. Microsc Res Tech, 2020, 83(9): 1025−1031. doi: 10.1002/jemt.23478
[44] Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip[J]. Lab Chip, 2008, 8(1): 98−106. doi: 10.1039/B713695A
[45] Mudanyali O, Tseng D, Oh C, et al. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications[J]. Lab Chip, 2010, 10(11): 1417−1428. doi: 10.1039/c000453g
[46] Bishara W, Su T W, Coskun A F, et al. Lensfree on-chip microscopy over a wide field-of-view using pixel super-resolution[J]. Opt Express, 2010, 18(11): 11181−11191. doi: 10.1364/OE.18.011181
[47] Jiang S W, Zhu J K, Song P M, et al. Wide-field, high-resolution lensless on-chip microscopy via near-field blind ptychographic modulation[J]. Lab Chip, 2020, 20(6): 1058−1065. doi: 10.1039/C9LC01027K
[48] Adams J K, Yan D, Wu J M, et al. In vivo lensless microscopy via a phase mask generating diffraction patterns with high-contrast contours[J]. Nat Biomed Eng, 2022, 6(5): 617−628. doi: 10.1038/s41551-022-00851-z
[49] Adams J K, Boominathan V, Avants B W, et al. Single-frame 3D fluorescence microscopy with ultraminiature lensless FlatScope[J]. Sci Adv, 2017, 3(12): e1701548. doi: 10.1126/sciadv.1701548
[50] Antipa N, Kuo G, Heckel R, et al. DiffuserCam: lensless single-exposure 3D imaging[J]. Optica, 2018, 5(1): 1−9. doi: 10.1364/optica.5.000001
[51] Kuo G, Liu F L, Grossrubatscher I, et al. On-chip fluorescence microscopy with a random microlens diffuser[J]. Opt Express, 2020, 28(6): 8384−8399. doi: 10.1364/OE.382055
[52] Tian F, Hu J J, Yang W J. GEOMScope: large field-of-view 3D lensless microscopy with low computational complexity[J]. Laser Photonics Rev, 2021, 15(8): 2100072. doi: 10.1002/lpor.202100072
[53] Zhou P, He H P, Ma H B, et al. A review of optical imaging technologies for microfluidics[J]. Micromachines, 2022, 13(2): 274. doi: 10.3390/MI13020274
[54] Gӧrӧcs Z, Tamamitsu M, Bianco V, et al. A deep learning-enabled portable imaging flow cytometer for cost-effective, high-throughput, and label-free analysis of natural water samples[J]. Light Sci Appl, 2018, 7(1): 66. doi: 10.1038/s41377-018-0067-0
[55] Song P M, Guo C F, Jiang S W, et al. Optofluidic ptychography on a chip[J]. Lab Chip, 2021, 21(23): 4549−4556. doi: 10.1039/D1LC00719J
[56] Zhu H Y, Mavandadi S, Coskun A F, et al. Optofluidic fluorescent imaging cytometry on a cell phone[J]. Anal Chem, 2011, 83(17): 6641−6647. doi: 10.1021/ac201587a
[57] Kim B, Oh S, Shin S, et al. Pumpless microflow cytometry enabled by viscosity modulation and immunobead labeling[J]. Anal Chem, 2018, 90(13): 8254−8260. doi: 10.1021/acs.analchem.8b01804
[58] Lee Y, Kim B, Choi S. Integrated microflow cytometry for portable immunophenotypic cell analysis[J]. Sensors Actuators A Phys, 2020, 309: 112038. doi: 10.1016/j.sna.2020.112038
[59] Wang B F, Li Y W, Zhou M F, et al. Smartphone-based platforms implementing microfluidic detection with image-based artificial intelligence[J]. Nat Commun, 2023, 14(1): 1341. doi: 10.1038/s41467-023-36017-x
[60] Sunny S, Baby A, James B L, et al. A smart tele-cytology point-of-care platform for oral cancer screening[J]. PLoS One, 2019, 14(11): e0224885. doi: 10.1371/journal.pone.0224885
[61] Zeinhom M M A, Wang Y J, Song Y, et al. A portable smart-phone device for rapid and sensitive detection of E. coli O157: H7 in Yoghurt and egg[J]. Biosens Bioelectron, 2018, 99: 479−485. doi: 10.1016/j.bios.2017.08.002
[62] Kanakasabapathy M K, Thirumalaraju P, Bormann C L, et al. Development and evaluation of inexpensive automated deep learning-based imaging systems for embryology[J]. Lab Chip, 2019, 19(24): 4139−4145. doi: 10.1039/C9LC00721K
[63] Bormann C L, Curchoe C L, Thirumalaraju P, et al. Correction to: deep learning early warning system for embryo culture conditions and embryologist performance in the ART laboratory[J]. J Assisted Reprod Genet, 2021, 38(7): 1893−1893. doi: 10.1007/s10815-021-02225-x
[64] Bormann C L, Kanakasabapathy M K, Thirumalaraju P, et al. Performance of a deep learning based neural network in the selection of human blastocysts for implantation[J]. Elife, 2020, 9: e55301. doi: 10.7554/eLife.55301
[65] Abraham E, Zhou J X, Liu Z W. Speckle structured illumination endoscopy with enhanced resolution at wide field of view and depth of field[J]. Opto Electron Adv, 2023, 6(7): 220163. doi: 10.29026/oea.2023.220163
[66] Chen Y W, He Y, Ye H, et al. Unified deep learning model for predicting fundus fluorescein angiography image from fundus structure image[J]. J Innovative Opt Health Sci, 2024, 17(3): 156−159. doi: 10.1142/S1793545824500032
[67] Xu X, Luo Q, Wang J X, et al. Large-field objective lens for multi-wavelength microscopy at mesoscale and submicron resolution[J]. Opto Electron Adv, 2024, 7(6): 230212. doi: 10.29026/oea.2024.230212
[68] 张子建, 王天义, 徐欣, 等. 偏振激光照明对多层薄膜结构成像对比度影响[J]. 光电工程, 2023, 50(7): 230089. doi: 10.12086/oee.2023.230089
Zhang Z J, Wang T Y, Xu X, et al. Effect of polarized laser illumination on imaging contrast of multilayer thin film structure[J]. Opto-Electron Eng, 2023, 50(7): 230089. doi: 10.12086/oee.2023.230089
[69] 张子建, 徐欣, 王吉祥, 等. 光片荧光显微镜研究进展[J]. 光电工程, 2023, 50(5): 220045. doi: 10.12086/oee.2023.220045
Zhang Z J, Xu X, Wang J X, et al. Review of the development of light sheet fluorescence microscopy[J]. Opto-Electron Eng, 2023, 50(5): 220045. doi: 10.12086/oee.2023.220045
[70] Gjeorgjievski M, Sarkar A, Shahid H, et al. Endoscopic full-thickness resection with reconstruction of the rectal wall[J]. Endoscopy, 2023, 55(S1): E133−E134. doi: 10.1055/A-1948-2007
[71] Hsia D W, Tanner N T, Shamblin C, et al. The latest generation in flexible bronchoscopes: a description and evaluation[J]. J Bronchol Interv Pulmonol, 2013, 20(4): 357−362. doi: 10.1097/LBR.0000000000000014
[72] Tomita Y, Yoshida N, Inoue K, et al. Two cases of colonic tumors observed by linked color imaging and texture and color enhancement imaging with the tablet-image comparison method[J]. DEN Open, 2021, 2(1): e47. doi: 10.1002/deo2.47
[73] Beltrán V P, Alonso-Lázaro N, Mansilla-Vivar R, et al. Single-operator cholangiopancreatoscopy in pancreatobiliary diseases: clinical experience in a tertiary referral hospital[J]. Rev Esp Enferm Dig, 2018, 110(12): 748−754. doi: 10.17235/reed.2018.5837/2018
[74] Wong C H K, Ko S, Wong O F, et al. A manikin study comparing the performance of the GlideScope®, the Airtraq® and the C-MAC® in endotracheal intubation using suction-assisted laryngoscopy airway decontamination techniques in a simulated massive haematemesis scenario by emergency doctors[J]. Hong Kong J Emerg Med, 2021, 28(5): 291−304. doi: 10.1177/1024907920957796
[75] Leon M G, Nguyen A, Nguyen A, et al. Diagnostic office hysteroscopy with the Storz TrophyScope® versus cooper surgical Endosee®[J]. Minerva Ginecol, 2020, 72(5): 310−315. doi: 10.23736/S0026-4784.20.04568-2
[76] Guo C L, Urner T, Jia S. 3D light-field endoscopic imaging using a GRIN lens array[J]. Appl Phys Lett, 2020, 116(10): 101105. doi: 10.1063/1.5143113
[77] Köhler T, Haase S, Bauer S, et al. ToF meets RGB: novel multi-sensor super-resolution for hybrid 3-D endoscopy[C]//16th International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), Nagoya, Japan, 2013: 139–146. https://doi.org/10.1007/978-3-642-40811-3_18.
[78] Stolyarov R, Buharin V, Val M, et al. Sub-millimeter precision 3D measurement through a standard endoscope with time of flight[C]//Proceedings of SPIE 11949, Advanced Biomedical and Clinical Diagnostic and Surgical Guidance Systems XX, San Francisco, 2022: 119490E. https://doi.org/10.1117/12.2605993.
[79] Maurice X, Albitar C, Doignon C, et al. A structured light-based laparoscope with real-time organs' surface reconstruction for minimally invasive surgery[C]//34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, 2012: 5769–5772. https://doi.org/10.1109/EMBC.2012.6347305.
[80] Kim D T, Cheng C H, Liu D G, et al. Designing a new endoscope for panoramic-view with focus-area 3D-vision in minimally invasive surgery[J]. J Med Biol Eng, 2020, 40(2): 204−219. doi: 10.1007/s40846-019-00503-9
[81] Zhou C J, Yu H, Yuan B, et al. Three-dimensional stitching of binocular endoscopic images based on feature points[J]. Photonics, 2021, 8(8): 330. doi: 10.3390/PHOTONICS8080330
[82] Yu H, Zhou C J, Zhang W, et al. A three-dimensional measurement method for binocular endoscopes based on deep learning[J]. Front Inf Technol Electron Eng, 2022, 23(4): 653−660. doi: 10.1631/FITEE.2000679
[83] Broxton M, Grosenick L, Yang S, et al. Wave optics theory and 3-D deconvolution for the light field microscope[J]. Opt Express, 2013, 21(21): 25418−25439. doi: 10.1364/OE.21.025418
[84] Levoy M, Ng R, Adams A, et al. Light field microscopy[J]. ACM Trans Graphics, 2006, 25(3): 924−934. doi: 10.1145/1141911.1141976
[85] Lee J S, Jung G S, Won Y H. Light field 3D endoscope based on electro-wetting lens array[C]//Proceedings of SPIE 10061, Microfluidics, BioMEMS, and Medical Microsystems XV, San Francisco, 2017: 100610J. https://doi.org/10.1117/12.2251660.
[86] Liu J D, Claus D, Xu T F, et al. Light field endoscopy and its parametric description[J]. Opt Lett, 2017, 42(9): 1804−1807. doi: 10.1364/OL.42.001804
[87] Beg S, Parra-Blanco A, Ragunath K. Optimising the performance and interpretation of small bowel capsule endoscopy[J]. Frontline Gastroenterol, 2018, 9(4): 300−308. doi: 10.1136/flgastro-2017-100878
[88] Nakajima F, Furumatsu Y, Yurugi T, et al. Investigation of small intestinal lesions in dialysis patients using capsule endoscopy[J]. Hemodial Int, 2019, 23(1): 77−80. doi: 10.1111/hdi.12683
[89] Ou G, Shahidi N, Galorport C, et al. Effect of longer battery life on small bowel capsule endoscopy[J]. World J Gastroenterol, 2015, 21(9): 2677−2682. doi: 10.3748/wjg.v21.i9.2677
[90] Cao Q, Deng R Y, Pan Y, et al. Robotic wireless capsule endoscopy: recent advances and upcoming technologies[J]. Nat Commun, 2024, 15(1): 4597. doi: 10.1038/s41467-024-49019-0
[91] Spada C, Piccirelli S, Hassan C, et al. AI-assisted capsule endoscopy reading in suspected small bowel bleeding: a multicentre prospective study[J]. Lancet Digital Health, 2024, 6(5): e345−e353. doi: 10.1016/S2589-7500(24)00048-7
[92] Inoue H, Igari T, Nishikage T, et al. A novel method of virtual histopathology using laser-scanning confocal microscopy in-vitro with untreated fresh specimens from the gastrointestinal mucosa[J]. Endoscopy, 2000, 32(6): 439−443. doi: 10.1055/s-2000-654
[93] Minsky M. Memoir on inventing the confocal scanning microscope[J]. Scanning, 1988, 10(4): 128−138. doi: 10.1002/sca.4950100403
[94] Ra H, Piyawattanametha W, Mandella M J, et al. Three-dimensional in vivo imaging by a handheld dual-axes confocal microscope[J]. Opt Express, 2008, 16(10): 7224−7232. doi: 10.1364/OE.16.007224
[95] Ra H, Piyawattanametha W, Gonzalez-Gonzalez E, et al. In vivo imaging of human and mouse skin with a handheld dual-axis confocal fluorescence microscope[J]. J Invest Dermatol, 2011, 131(5): 1061−1066. doi: 10.1038/jid.2010.401
[96] Hwang K, Seo Y H, Kim D Y, et al. Handheld endomicroscope using a fiber-optic harmonograph enables real-time and in vivo confocal imaging of living cell morphology and capillary perfusion[J]. Microsyst Nanoeng, 2020, 6(1): 72. doi: 10.1038/s41378-020-00182-6
[97] Goetz M, Deris I, Vieth M, et al. Near-infrared confocal imaging during mini-laparoscopy: a novel rigid endomicroscope with increased imaging plane depth[J]. J Hepatol, 2010, 53(1): 84−90. doi: 10.1016/j.jhep.2010.01.039
[98] Dickensheets D L, Kino G S. Scanned optical fiber confocal microscope[C]//Proceedings of SPIE 2184, Three-Dimensional Microscopy: Image Acquisition and Processing, San Jose, 1994: 39–47. https://doi.org/10.1117/12.172104.
[99] Seo Y H, Hwang K, Park H C, et al. Electrothermal MEMS fiber scanner for optical endomicroscopy[J]. Opt Express, 2016, 24(4): 3903−3909. doi: 10.1364/OE.24.003903
[100] Seibel E J, Smithwick Q Y J. Unique features of optical scanning, single fiber endoscopy[J]. Lasers Surg Med, 2002, 30(3): 177−183. doi: 10.1002/lsm.10029
[101] Myaing M T, MacDonald D J, Li X D. Fiber-optic scanning two-photon fluorescence endoscope[J]. Opt Lett, 2006, 31(8): 1076−1078. doi: 10.1364/OL.31.001076
[102] Rivera D R, Brown C M, Ouzounov D G, et al. Compact and flexible raster scanning multiphoton endoscope capable of imaging unstained tissue[J]. Proc Natl Acad Sci USA, 2011, 108(43): 17598−17603. doi: 10.1073/pnas.1114746108
[103] Laemmel E, Genet M, Le Goualher G, et al. Fibered confocal fluorescence microscopy (Cell-viZio™) facilitates extended imaging in the field of microcirculationa comparison with intravital microscopy[J]. J Vasc Res, 2004, 41(5): 400−411. doi: 10.1159/000081209
[104] Sun J T, Shu C H, Appiah B, et al. Needle-compatible single fiber bundle image guide reflectance endoscope[J]. J Biomed Opt, 2010, 15(4): 040502. doi: 10.1117/1.3465558
[105] Wang J F, Li H, Tian G, et al. Near-infrared probe-based confocal microendoscope for deep-tissue imaging[J]. Biomed Opt Express, 2018, 9(10): 5011−5025. doi: 10.1364/BOE.9.005011
[106] Li H, Hao Z Y, Huang J F, et al. 500 μm field-of-view probe-based confocal microendoscope for large-area visualization in the gastrointestinal tract[J]. Photonics Res, 2021, 9(9): 1829−1841. doi: 10.1364/PRJ.431767
[107] Huang D, Swanson E A, Lin C P, et al. Optical coherence tomography[J]. Science, 1991, 254(5035): 1178−1181. doi: 10.1126/science.1957169
[108] 刘德军, 黄梓毅, 李卓荣, 等. 光学相干层析显微内窥成像技术研究进展(特邀)[J]. 激光与光电子学进展, 2024, 61(2): 0211025. doi: 10.3788/LOP232208
Liu D J, Huang Z Y, Li Z R, et al. Recent advances in micro-endoscopies based on optical coherence tomography (invited)[J]. Laser Optoelectron Prog, 2024, 61(2): 0211025. doi: 10.3788/LOP232208
[109] Ge X, Chen S, Lin K, et al. Deblurring, artifact-free optical coherence tomography with deconvolution-random phase modulation[J]. Opto Electron Sci, 2024, 3(1): 230020. doi: 10.29026/oes.2024.230020
[110] Huang J J, Fan J Y, He Y, et al. Physical compensation method for dispersion of multiple materials in swept source optical coherence tomography[J]. J Biophotonics, 2023, 16(10): e202300167. doi: 10.1002/jbio.202300167
[111] Kang J Q, Zhu R, Sun Y X, et al. Pencil-beam scanning catheter for intracoronary optical coherence tomography[J]. Opto Electron Adv, 2022, 5(3): 200050. doi: 10.29026/oea.2022.200050
[112] Wang J, Zong Y, He Y, et al. Domain adaptation-based automated detection of retinal diseases from optical coherence tomography images[J]. Curr Eye Res, 2023, 48(9): 836−842. doi: 10.1080/02713683.2023.2212878
[113] 李云耀, 樊金宇, 蒋天亮, 等. 光学相干层析技术在眼科手术导航方面的研究进展[J]. 光电工程, 2023, 50(1): 220027. doi: 10.12086/oee.2023.220027
Li Yunyao, Fan Jinyu, Jiang Tianliang, et al. Review of the development of optical coherence tomography imaging navigation technology in ophthalmic surgery[J]. Opto-Electron Eng, 2023, 50(1): 220027. doi: 10.12086/oee.2023.220027
[114] 杨建文, 黄江杰, 何益, 等. 线聚焦谱域光学相干层析成像的分段色散补偿像质优化方法[J]. 光电工程, 2024, 51(6): 240042. doi: 10.12086/oee.2024.240042
Yang J W, Huang J J, He Y, et al. Image quality optimization of line-focused spectral domain optical coherence tomography with subsection dispersion compensation[J]. Opto-Electron Eng, 2024, 51(6): 240042. doi: 10.12086/oee.2024.240042
[115] Tearney G J, Boppart S A, Bouma B E, et al. Scanning single-mode fiber optic catheter-endoscope for optical coherence tomography[J]. Opt Lett, 1996, 21(7): 543−545. doi: 10.1364/OL.21.000543
[116] Tearney G J, Brezinski M E, Bouma B E, et al. In vivo endoscopic optical biopsy with optical coherence tomography[J]. Science, 1997, 276(5321): 2037−2039. doi: 10.1126/science.276.5321.2037
[117] Yin B W, Chu K K, Liang C P, et al. μOCT imaging using depth of focus extension by self-imaging wavefront division in a common-path fiber optic probe[J]. Opt Express, 2016, 24(5): 5555−5564. doi: 10.1364/OE.24.005555
[118] Yin B W, Hyun C, Gardecki J A, et al. Extended depth of focus for coherence-based cellular imaging[J]. Optica, 2017, 4(8): 959−965. doi: 10.1364/OPTICA.4.000959
[119] Yin B W, Piao Z L, Nishimiya K, et al. 3D cellular-resolution imaging in arteries using few-mode interferometry[J]. Light Sci Appl, 2019, 8: 104. doi: 10.1038/s41377-019-0211-5
[120] Li X D, Chudoba C, Ko T, et al. Imaging needle for optical coherence tomography[J]. Opt Lett, 2000, 25(20): 1520−1522. doi: 10.1364/OL.25.001520
[121] Lorenser D, Yang X, Kirk R W, et al. Ultrathin side-viewing needle probe for optical coherence tomography[J]. Opt Lett, 2011, 36(19): 3894−3896. doi: 10.1364/OL.36.003894
[122] Mao Y, Chang, S, Flueraru, C. Fiber lenses for ultra-small probes used in optical coherent tomography[J]. Journal of Biomedical Science and Engineering, 2010, 3(1): 27−34. doi: 10.4236/jbise.2010.31004
[123] Yuan W, Brown R, Mitzner W, et al. Super-achromatic monolithic microprobe for ultrahigh-resolution endoscopic optical coherence tomography at 800 nm[J]. Nat Commun, 2017, 8(1): 1531. doi: 10.1038/s41467-017-01494-4
[124] Xing J C, Kim J, Yoo H. Design and fabrication of an optical probe with a phase filter for extended depth of focus[J]. Opt Express, 2016, 24(2): 1037−1044. doi: 10.1364/OE.24.001037
[125] Pahlevaninezhad H, Khorasaninejad M, Huang Y W, et al. Nano-optic endoscope for high-resolution optical coherence tomography in vivo[J]. Nat Photonics, 2018, 12(9): 540−547. doi: 10.1038/s41566-018-0224-2
[126] Chen S Y, Wang Z C, Zhang D, et al. Miniature fluorescence microscopy for imaging brain activity in freely-behaving animals[J]. Neurosci Bull, 2020, 36(10): 1182−1190. doi: 10.1007/s12264-020-00561-z
[127] Dombeck D A, Harvey C D, Tian L, et al. Functional imaging of hippocampal place cells at cellular resolution during virtual navigation[J]. Nat Neurosci, 2010, 13(11): 1433−1440. doi: 10.1038/nn.2648
[128] Flusberg B A, Cocker E D, Piyawattanametha W, et al. Fiber-optic fluorescence imaging[J]. Nat Methods, 2005, 2(12): 941−950. doi: 10.1038/nmeth820
[129] 刘晓宇, 刘紫千, 斯科, 等. 微型化显微成像系统的关键技术及研究进展(特邀)[J]. 激光与光电子学进展, 2024, 61(2): 0211009. doi: 10.3788/LOP232709
Liu X Y, Liu Z Q, Si K, et al. Key technologies and progresses of miniaturized microscopic imaging system (invited)[J]. Laser Optoelectron Prog, 2024, 61(2): 0211009. doi: 10.3788/LOP232709
[130] Ghosh K K, Burns L D, Cocker E D, et al. Miniaturized integration of a fluorescence microscope[J]. Nat Methods, 2011, 8(10): 871−878. doi: 10.1038/nmeth.1694
[131] Cai D J, Aharoni D, Shuman T, et al. A shared neural ensemble links distinct contextual memories encoded close in time[J]. Nature, 2016, 534(7605): 115−118. doi: 10.1038/nature17955
[132] Liberti W A, Perkins L N, Leman D P, et al. An open source, wireless capable miniature microscope system[J]. J Neural Eng, 2017, 14(4): 045001. doi: 10.1088/1741-2552/aa6806
[133] Guo C L, Blair G J, Sehgal M, et al. Miniscope-LFOV: a large-field-of-view, single-cell-resolution, miniature microscope for wired and wire-free imaging of neural dynamics in freely behaving animals[J]. Sci Adv, 2023, 9(16): eadg3918. doi: 10.1126/sciadv.adg3918
[134] Rynes M L, Surinach D A, Linn S, et al. Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice[J]. Nat Methods, 2021, 18(4): 417−425. doi: 10.1038/s41592-021-01104-8
[135] Shekhtmeyster P, Duarte D, Carey E M, et al. Trans-segmental imaging in the spinal cord of behaving mice[J]. Nat Biotechnol, 2023, 41(12): 1729−1733. doi: 10.1038/s41587-023-01700-3
[136] Ozbay B N, Losacco J T, Cormack R, et al. Miniaturized fiber-coupled confocal fluorescence microscope with an electrowetting variable focus lens using no moving parts[J]. Opt Lett, 2015, 40(11): 2553−2556. doi: 10.1364/OL.40.002553
[137] Dussaux C, Szabo V, Chastagnier Y, et al. Fast confocal fluorescence imaging in freely behaving mice[J]. Sci Rep, 2018, 8(1): 16262. doi: 10.1038/s41598-018-34472-x
[138] Supekar O D, Sias A, Hansen S R, et al. Miniature structured illumination microscope for in vivo 3D imaging of brain structures with optical sectioning[J]. Biomed Opt Express, 2022, 13(4): 2530−2541. doi: 10.1364/BOE.449533
[139] Bagramyan A, Tabourin L, Rastqar A, et al. Focus-tunable microscope for imaging small neuronal processes in freely moving animals[J]. Photonics Res, 2021, 9(7): 1300−1309. doi: 10.1364/PRJ.418154
[140] Yanny K, Antipa N, Liberti W, et al. Miniscope3D: optimized single-shot miniature 3D fluorescence microscopy[J]. Light Sci Appl, 2020, 9(1): 171. doi: 10.1038/s41377-020-00403-7
[141] Sawinski J, Wallace D J, Greenberg D S, et al. Visually evoked activity in cortical cells imaged in freely moving animals[J]. Proc Natl Acad Sci USA, 2009, 106(46): 19557−19562. doi: 10.1073/pnas.0903680106
[142] Zong W J, Wu R L, Li M L, et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice[J]. Nat Methods, 2017, 14(7): 713−719. doi: 10.1038/nmeth.4305
[143] Zong W J, Wu R L, Chen S Y, et al. Miniature two-photon microscopy for enlarged field-of-view, multi-plane and long-term brain imaging[J]. Nat Methods, 2021, 18(1): 46−49. doi: 10.1038/s41592-020-01024-z
[144] Zhao C Z, Chen S Y, Zhang L F, et al. Miniature three-photon microscopy maximized for scattered fluorescence collection[J]. Nat Methods, 2023, 20(4): 617−622. doi: 10.1038/s41592-023-01777-3
[145] Yu N F, Genevet P, Kats M A, et al. Light propagation with phase discontinuities: generalized laws of reflection and refraction[J]. Science, 2011, 334(6054): 333−337. doi: 10.1126/science.1210713
[146] Arbabi E, Li J Q, Hutchins R J, et al. Two-photon microscopy with a double-wavelength metasurface objective lens[J]. Nano Lett, 2018, 18(8): 4943−4948. doi: 10.1021/acs.nanolett.8b01737
[147] Wang C H, Chen Q M, Liu H L, et al. Miniature two-photon microscopic imaging using dielectric metalens[J]. Nano Lett, 2023, 23(17): 8256−8263. doi: 10.1021/acs.nanolett.3c02439
[148] Ren H R, Jang J, Li C H, et al. An achromatic metafiber for focusing and imaging across the entire telecommunication range[J]. Nat Commun, 2022, 13(1): 4183. doi: 10.1038/s41467-022-31902-3
[149] Jin L H, Liu B, Zhao F Q, et al. Deep learning enables structured illumination microscopy with low light levels and enhanced speed[J]. Nat Commun, 2020, 11(1): 1934. doi: 10.1038/s41467-020-15784-x
[150] Zhou H, Cai R Y, Quan T W, et al. 3D high resolution generative deep-learning network for fluorescence microscopy imaging[J]. Opt Lett, 2020, 45(7): 1695−1698. doi: 10.1364/OL.387486
[151] Falk T, Mai D, Bensch R, et al. U-Net: deep learning for cell counting, detection, and morphometry[J]. Nat Methods, 2019, 16(1): 67−70. doi: 10.1038/s41592-018-0261-2
[152] Todorov M I, Paetzold J C, Schoppe O, et al. Machine learning analysis of whole mouse brain vasculature[J]. Nat Methods, 2020, 17(4): 442−449. doi: 10.1038/s41592-020-0792-1
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: