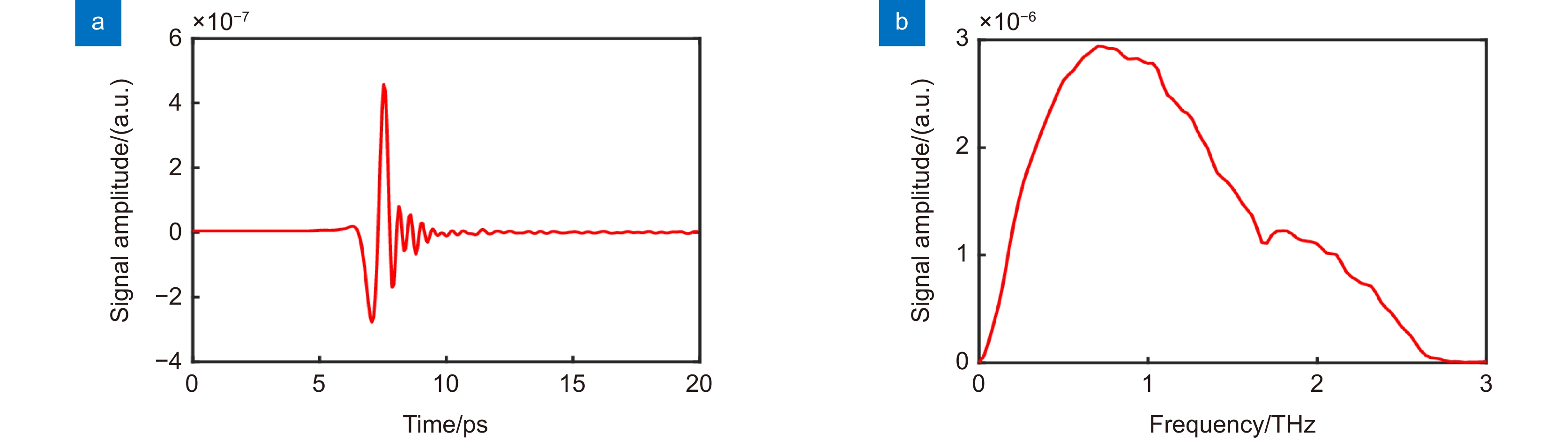
-
摘要
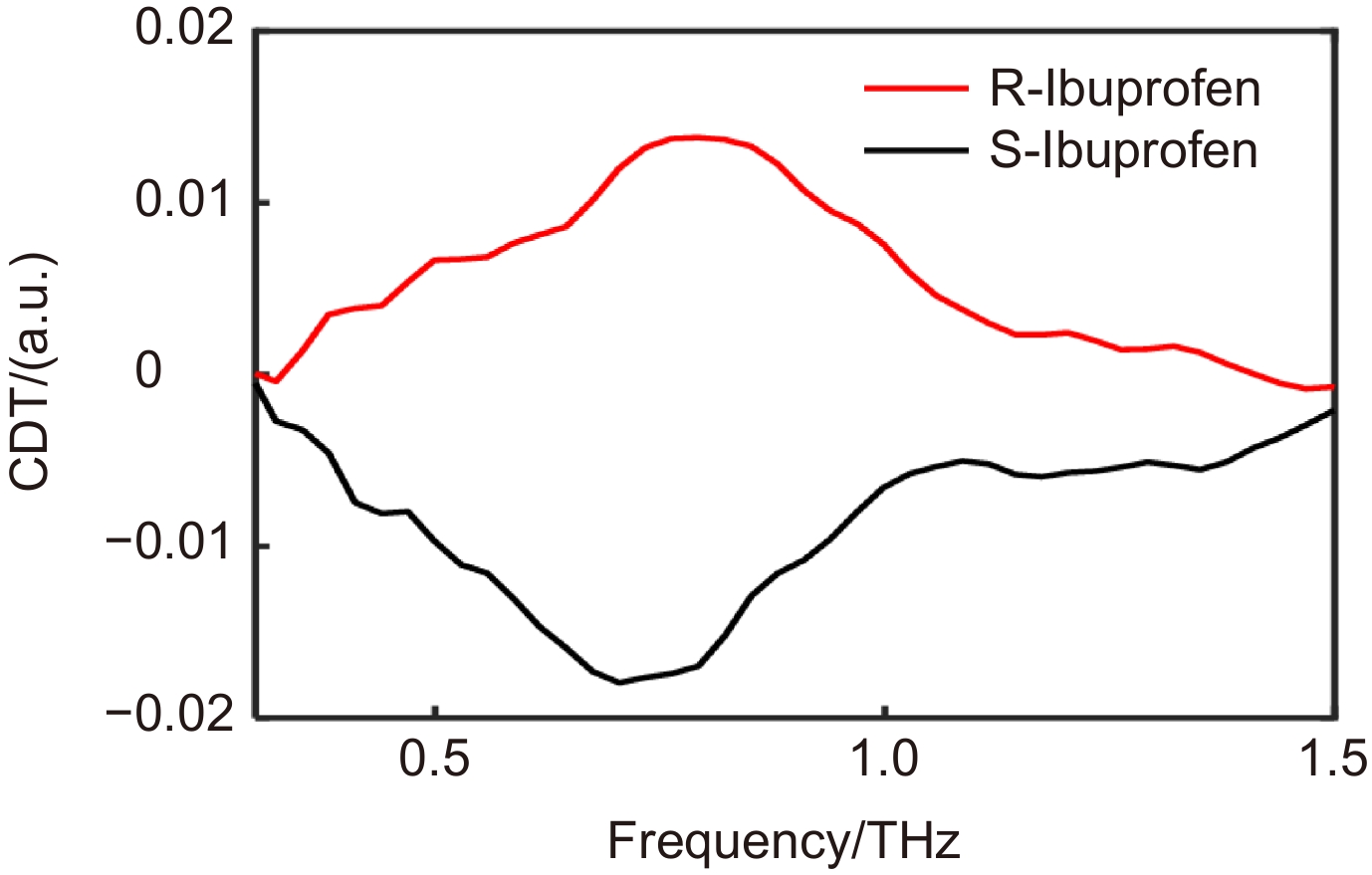
不同手性的生物分子具有不同甚至相反的生物和药理活性。由于很多生物大分子的振动和转动能级分布在太赫兹波段,使得太赫兹波谱技术成为生物大分子识别的有效手段,但是太赫兹时域光谱系统都采用线偏振光源,无法对手性分子进行有效识别。我们在理论上利用线偏振的琼斯矩阵模型合成圆偏振下的琼斯矩阵,根据圆偏光的透射率差异性进一步计算样品的透射圆二色光谱,为表征不同手性分子提供了一种有效方法。基于透射式太赫兹时域光谱系统,对(R)-(-)-Ibuprofen和(S)-(+)-Ibuprofen的光谱进行测试,计算了(R)-(-)-Ibuprofen和(S)-(+)-Ibuprofen的线偏透过率和圆偏透过率;并计算出透射性圆二色光谱,两种手性物质的圆二色性值达到0.015,有效地实现了对(R)-(-)-Ibuprofen和(S)-(+)-Ibuprofen的识别效果。这一方法为利用太赫兹光谱技术检测和识别手性分子提供了参考。
Abstract
Biomolecules with different chirality have different or even opposite biological and pharmacological activities. Since vibration and rotation energy levels of many biomolecules lie within the terahertz range, terahertz spectroscopy has emerged as a useful tool for biomolecular identification. Nevertheless, linearly polarized light sources are used in terahertz time-domain spectroscopy, which is ineffective for identifying chiral compounds. We theoretically constructed a circularly polarized Jones matrix using a linearly polarized Jones matrix model. We also calculated the transmission circular dichroism spectrum of the sample based on the difference in transmittance of circularly polarized light, offering a useful technique for describing various chiral compounds. The spectra of (R)-(-)-Ibuprofen and (S)-(+)-Ibuprofen were investigated using a transmission terahertz time-domain spectroscopy system, and the linear polarization biased transmittance and circular polarization transmittance of (R)-(-)-Ibuprofen and (S)-(+)-Ibuprofen were computed. Additionally, the transmittance circular dichroic spectra were calculated, and the two chiral compounds' circular dichroic values reached 0.015, successfully achieving the (R)-(-)-Ibuprofen and (S)-(+)-Ibuprofen recognition effect. This technique serves as a guide for chiral molecule identification and detection using terahertz spectroscopy technology.
-
Key words:
- Jones matrix /
- circular dichroism /
- terahertz /
- chiral recognition
-
Overview
Overview: Chiral molecules are defined as materials that have chiral centers, also referred to as asymmetric centers in chemical structure. Biomolecules with different chirality exhibit distinct or even opposing biological and pharmacological effects, even when their structural groups are the same. Chirality detection and recognition have long been a significant issue in the realm of life sciences. Circular dichroism, a method of identifying chiral substances, is produced when left- and right-handed circularly polarized light are absorbed differently by different chiral molecules. This results in different amplitudes of circularly polarized light passing through different rotations. Currently, available techniques for detecting chiral drugs include high performance liquid chromatography, chiral Raman spectroscopy, nuclear magnetic resonance spectroscopy, and polarimeter detection; nevertheless, there are drawbacks, such as costly and difficult-to-use procedures. The terahertz spectroscopy technique becomes an excellent way to identify biological macromolecules since many of them have vibrational and rotational energy levels that fall in the terahertz range. Currently, linearly polarized light sources are used in the terahertz time-domain spectroscopy system, which is unable to detect chiral compounds. Theoretically, we can synthesize the Jones matrix under circular polarization using the Jones matrix model of linear polarization. We can then determine the circular dichroism spectrum of the sample based on the transmission difference of circular polarization light. This approach offers a useful way to characterize various chiral molecules. By rotating the crystal and the experimental sample through four measurements, the time-domain spectra under various polarization states are obtained based on the transmitted-terahertz time-domain spectroscopy system. The Jones matrix of the sample online polarization space is obtained, which is then converted into the circular polarization space. The circular polarization space's Jones matrix component is utilized to compute the sample's circular dichroism (CD) spectrum. This study presents the measurement of the spectra of (R)-(-)-Ibuprofen and (S)-(+)-Ibuprofen, together with the calculation of their linear and circular bias transmittances. The circular dichroism spectra of the two chiral substances are calculated, and the circular dichroism values of the two chiral substances reach 0.015, which effectively realizes the recognition effect of (R)-(-)-Ibuprofen and (S)-(+)-Ibuprofen. This work provides a reference for the following chiral recognition in the terahertz band by using the superstructure surface and improving the local intensity of the light field through high-quality resonance to improve the accuracy and sensitivity of chiral recognition.
-

-
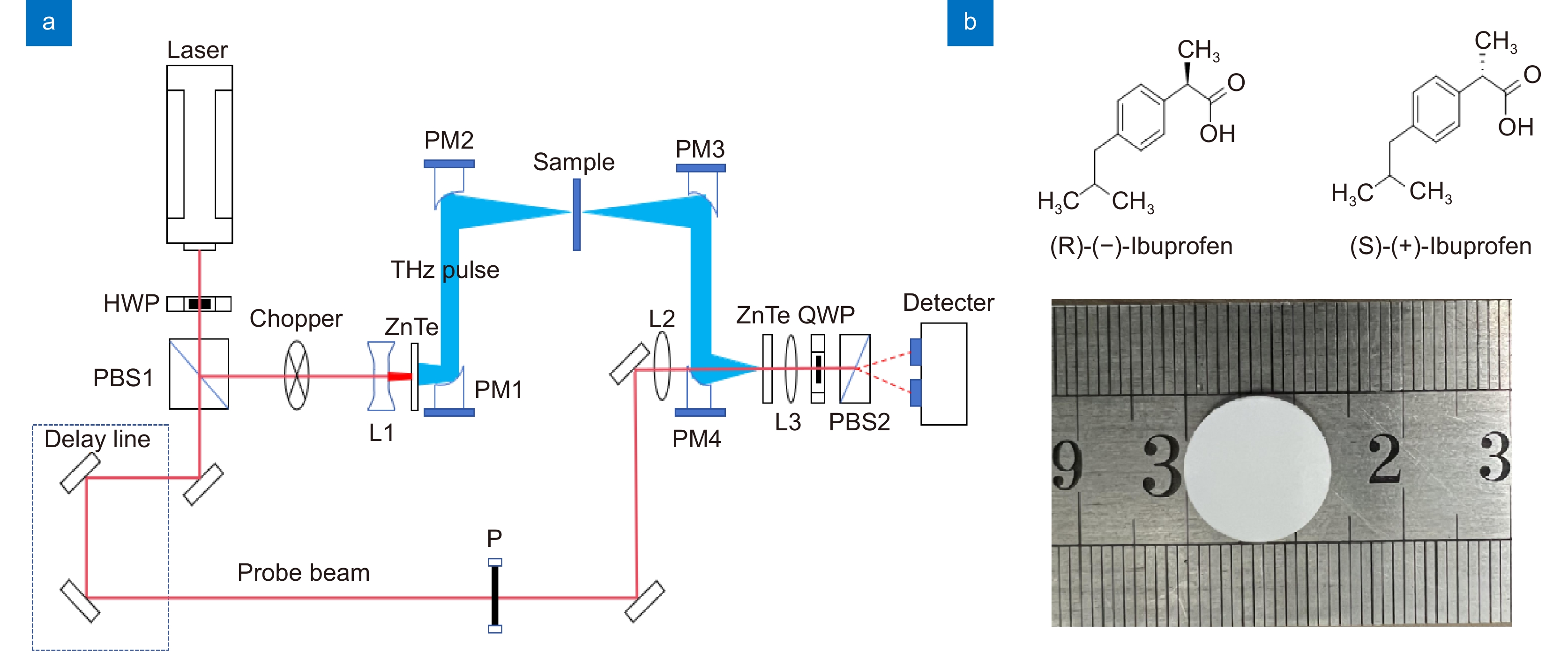
图 1 (a) 透射式太赫兹时域光谱系统示意图。其中HWP表示半波片,PBS表示偏振分光棱镜,L表示透镜,PM表示抛面镜,QWP表示四分之一玻片,P表示偏振片。(b) 实验压片样品及其分子式
Figure 1. (a) Schematic diagram of transmissive terahertz time-domain spectroscopy. HWP: half-wave plate, PBS: polarization splitting prism, L: lens, PM: parabolic mirror, QWP: quarter wave plate, and P: polarizer; (b) Experimental tablet sample and corresponding molecular formula
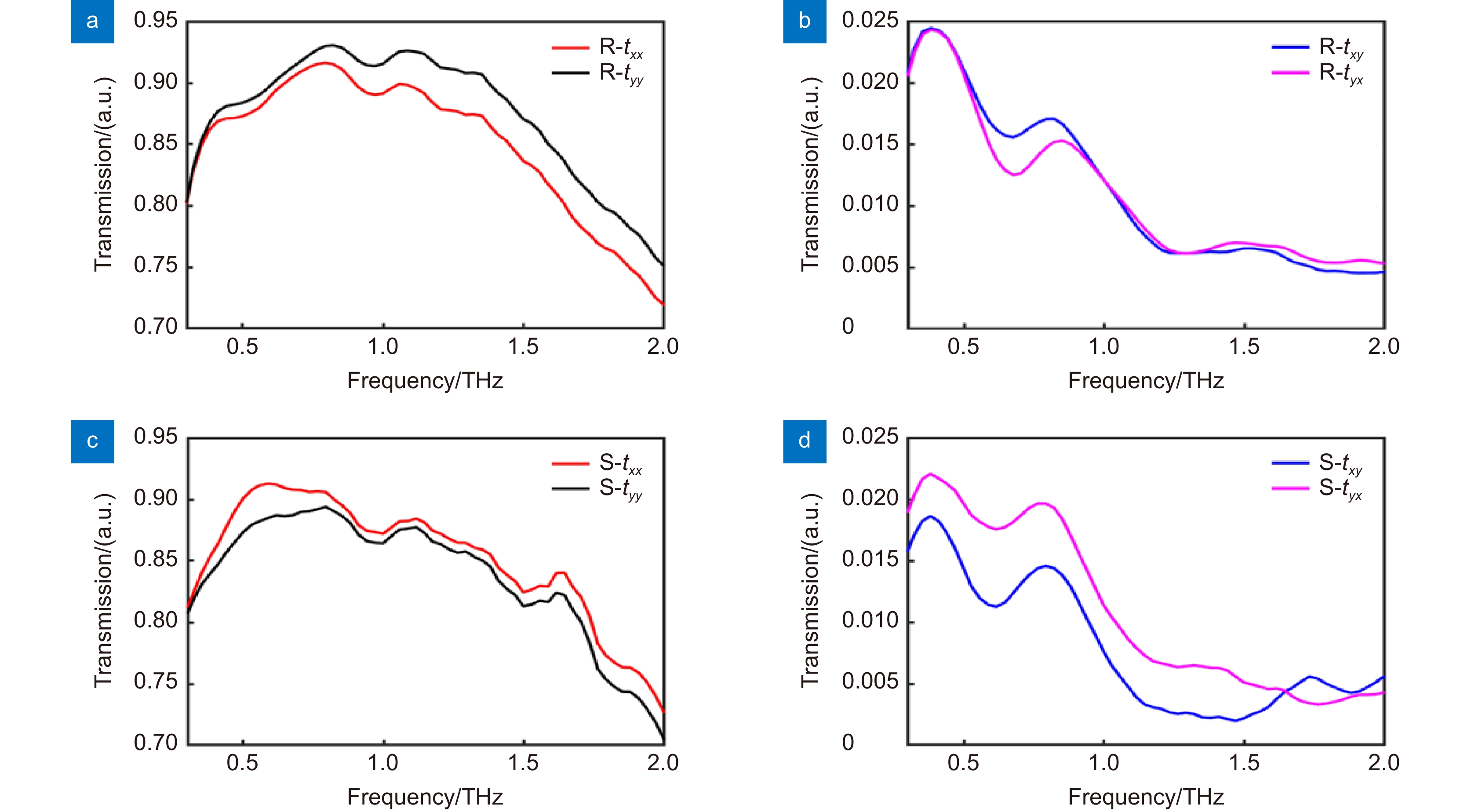
图 3 (R)-(-)-Ibuprofen和(S)-(+)-Ibuprofen的线偏振透过率。(a) (R)-(-)-Ibuprofen的同线偏透过率;(b) (R)-(-)-Ibuprofen的交叉线偏透过率;(c) (S)-(+)-Ibuprofen的同线偏透过率;(d) (S)-(+)-Ibuprofen的交叉线偏透过率
Figure 3. Transmittances of (R)-(-)-ibuprofen and (S)-(+) -ibuprofen for linear polarized terahertz wave. (a) Transmittances of (R)-(-)-Ibuprofen for same linear polarization; (b) Transmittances of (R)-(-)-Ibuprofen for cross linear polarization; (c) Transmittances of (S)-(+)-Ibuprofen for same linear polarization; (d) Transmittance of (S)-(+)-Ibuprofen for cross linear polarization
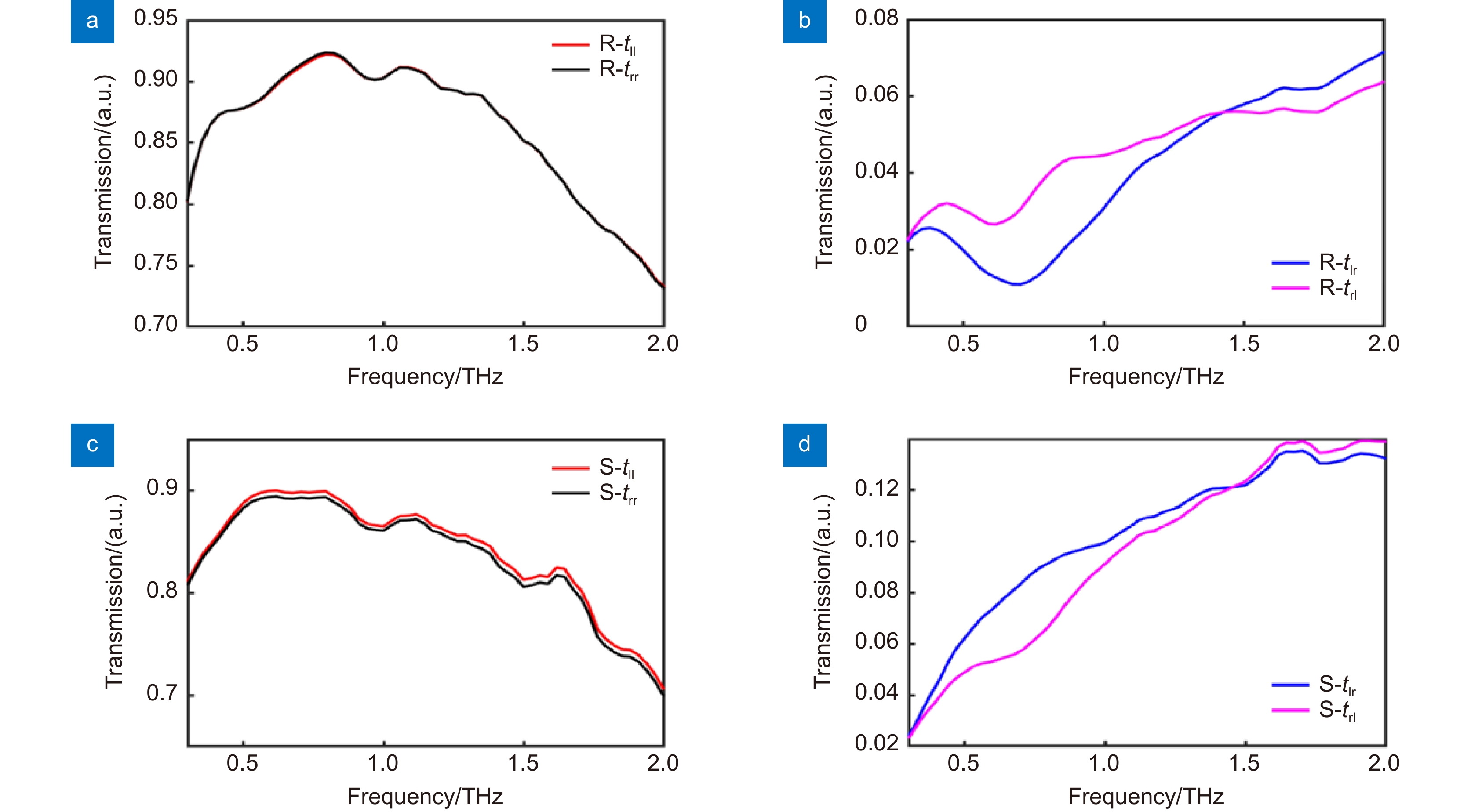
图 4 (R)-(-)-Ibuprofen和(S)-(+)-Ibuprofen的圆偏振透过率。(a) (R)-(-)-Ibuprofen的同圆偏透过率;(b) (R)-(-)-Ibuprofen的交叉圆偏透过率;(c) (S)-(+)-Ibuprofen的同圆偏透过率;(d) (S)-(+)-Ibuprofen的交叉圆偏透过率
Figure 4. Transmittances of (R)-(-)-Ibuprofen and (S)-(+) -ibuprofen for circular polarized wave. (a) Transmittances of (R)-(-)-Ibuprofen for same circular polarization; (b) Transmittance of (R)-(-)-Ibuprofen for cross circular polarization; (c) Transmittance of (S)-(+)-Ibuprofen for same circular polarization; (d) Transmittance of (S)-(+)-Ibuprofen for cross circular polarization
-
参考文献
[1] Wagnière G H. On Chirality and the Universal Asymmetry: Reflections on Image and Mirror Image[M]. Zürich: Verlag Helvetica Chimica Acta, 2007: 1–247.
[2] 蓝翔, 邓钦荣, 张汶婷, 等. 基于扭转悬链线结构的高效手性吸波器[J]. 光电工程, 2022, 49(10): 220157. doi: 10.12086/oee.2022.220157
Lan X, Deng Q R, Zhang W T, et al. Efficient chiral absorber based on twisted catenary structure[J]. Opto-Electron Eng, 2022, 49(10): 220157. doi: 10.12086/oee.2022.220157
[3] Mason S F. Optical Activity and Chiral Discrimination[M]. Berlin: Springer, 2013: 1–372.
[4] Blakemore C, Jennett S. The Oxford Companion to the Body[M]. Oxford: Oxford University Press, 2001: 76–78.
[5] Shi W N, Fan F, Zhang Z Y, et al. Terahertz sensing for r/s chiral ibuprofen via all-dielectric metasurface with higher-order resonance[J]. Appl Sci, 2021, 11(19): 8892. doi: 10.3390/app11198892
[6] 张林群, 李颖, 杨红晓. 圆二色光谱仪和旋光仪对手性药物比旋度测定的比较[J]. 分析仪器, 2014, (6): 64−68. doi: 10.3969/j.issn.1001-232x.2014.06.014
Zhang L Q, Li Y, Yang X H. Comparision of specifical rotation detection of chiral medicine between circular dichroism spectrometer and polarimeter[J]. Anal Instrument, 2014, (6): 64−68. doi: 10.3969/j.issn.1001-232x.2014.06.014
[7] Kumar P, Simon A, Kotov N A. Enantiomeric discrimination by chiral electromagnetic resonance enhancement[J]. Chirality, 2023, 35(10): 732−738. doi: 10.1002/chir.23578
[8] Fan M K, Andrade G F S, Brolo A G. A review on the fabrication of substrates for surface enhanced Raman spectroscopy and their applications in analytical chemistry[J]. Anal Chim Acta, 2011, 693(1-2): 7−25. doi: 10.1016/j.aca.2011.03.002
[9] Kong H J, Sun X P, Yang L, et al. Chirality detection by Raman spectroscopy: the case of enantioselective interactions between amino acids and polymer-modified chiral silica[J]. Anal Chem, 2020, 92(21): 14292−14296. doi: 10.1021/acs.analchem.0c03286
[10] Lv C G, Zhou Z Q. Chiral HPLC separation and absolute configuration assignment of a series of new triazole compounds[J]. J Sep Scince, 2011, 34(4): 363−370. doi: 10.1002/jssc.201000762
[11] Yang X, Zhao X, Yang K, et al. Biomedical applications of terahertz spectroscopy and imaging[J]. Trends Biotechnol, 2016, 34(10): 810−824. doi: 10.1016/j.tibtech.2016.04.008
[12] Chen Y X, Zhang F Y, Dang Z B, et al. Chiral detection of biomolecules based on reinforcement learning[J]. Opto-Electron Sci, 2023, 2(1): 220019. doi: 10.29026/oes.2023.220019
[13] Xu W T, Xie L J, Ying Y B. Mechanisms and applications of terahertz metamaterial sensing: a review[J]. Nanoscale, 2017, 9(37): 13864−13878. doi: 10.1039/C7NR03824K
[14] Seo M, Park H R. Terahertz biochemical molecule-specific sensors[J]. Adv Opt Mater, 2020, 8(3): 1900662. doi: 10.1002/adom.201900662
[15] Li K X, Li D, Zhang Y. Terahertz spectral properties of 5-substituted uracils[J]. Sensors, 2021, 21(24): 8292. doi: 10.3390/s21248292
[16] Zhang Z Y, Zhong C Z, Fan F, et al. Terahertz polarization and chirality sensing for amino acid solution based on chiral metasurface sensor[J]. Sens Actuat B Chem, 2020, 330: 129315. doi: 10.1016/j.snb.2020.129315
[17] Wu Z P, Zhu Z J, Cheng C, et al. Terahertz spectroscopy of enantiomeric and racemic pyroglutamic acid[J]. Spectrochim Acta Part A Mol Biomol Spectrosc, 2020, 225: 117509. doi: 10.1016/j.saa.2019.117509
[18] Bian Y J, Zhu Z Q, Zhang X, et al. Terahertz spectroscopy for quantitatively elucidating the crystal transformation of chiral histidine enantiomers to racemic compounds[J]. Food Chem, 2023, 406: 135043. doi: 10.1016/j.foodchem.2022.135043
[19] Wang Z F, Peng Y, Shi C J, et al. Qualitative and quantitative recognition of chiral drugs based on terahertz spectroscopy[J]. Analyst, 2021, 146(12): 3888−3898. doi: 10.1039/D1AN00500F
[20] Liu J Y, Zhang T R, Tan Z Y, et al. Chiral enantiomer recognition of amino acids enhanced by terahertz spin beam separation based on a Pancharatnam-Berry metasurface[J]. Opt Lett, 2023, 48(2): 440−443. doi: 10.1364/OL.477839
[21] Shi W N, Wang Y M, Fan F, et al. THz enantiomers of drugs recognized by the polarization enhancement of gold nanoparticles on an asymmetric metasurface[J]. Nanoscale, 2023, 15(34): 14146−14154. doi: 10.1039/D3NR01826A
[22] Kim J, Yang Y, Badloe T, et al. Geometric and physical configurations of meta-atoms for advanced metasurface holography[J]. InfoMat, 2021, 3(7): 739−754. doi: 10.1002/inf2.12191
[23] 侯春鹤, 朱运东, 李丽娟, 等. 太赫兹时域光谱技术的参数提取及其误差分析[J]. 光电工程, 2018, 45(2): 170534. doi: 10.12086/oee.2018.170534
Hou C H, Zhu Y D, Li L J, et al. Optical parameter extraction and error analysis of terahertz time domain spectrum detection[J]. Opto-Electron Eng, 2018, 45(2): 170534. doi: 10.12086/oee.2018.170534
[24] Li J T, Wang G C, Yue Z, et al. Dynamic phase assembled terahertz metalens for reversible conversion between linear polarization and arbitrary circular polarization[J]. Opto-Electron Adv, 2022, 5(1): 210062. doi: 10.29026/oea.2022.210062
[25] 史卓琳, 贺景琳, 王金金, 等. 基于相变材料GST的圆二色性可调谐外在手征超表面设计[J]. 光电工程, 2022, 49(10): 220092. doi: 10.12086/oee.2022.220092
Shi Z L, He J L, Wang J J, et al. Design of tunable circular dichroism extrinsic chiral metasurface based on phase change material GST[J]. Opto-Electron Eng, 2022, 49(10): 220092. doi: 10.12086/oee.2022.220092
[26] Chen Y, Chen W J, Kong X H, et al. Can weak chirality induce strong coupling between resonant states?[J]. Phys Rev Lett, 2022, 128(14): 146102. doi: 10.1103/PhysRevLett.128.146102
[27] Ali A, Khaliq H S, Asad A, et al. Dielectric chiral metasurfaces for enhanced circular dichroism spectroscopy at near infrared regime[J]. RSC Adv, 2023, 13(30): 20958−20965. doi: 10.1039/D3RA02331A
[28] 胡维东, 杜响, 刘思玉, 等. 基于准连续域束缚态介质超表面的光微流折射率传感研究[J]. 光电工程, 2023, 50(9): 230124. doi: 10.12086/oee.2023.230124
Hu W D, Du X, Liu S Y, et al. Optofluidic refractometric sensor based on quasi-bound states in the continuum in all-dielectric metasurface[J]. Opto-Electron Eng, 2023, 50(9): 230124. doi: 10.12086/oee.2023.230124
-
访问统计


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: