-
摘要
激光微细加工技术具有超快、超精密等特性,在医疗器材领域的应用中有着传统加工技术无可比拟的独特优势,尤其是对生物材料表面加工改性,提高材料生物相容性方面有着不可替代的作用。本文综述了近年来激光微加工技术在医疗器材制造加工领域的最新应用,着重介绍了血管支架和骨支架的结构与表面制造,生物材料表面改性与抗菌性处理等。最后对目前激光微加工技术存在的局限性做了讨论,对未来激光微加工技术在医疗器材领域的应用发展做了展望。
Abstract
Laser microfabrication has the characteristics of ultra fast, ultra precision, etc. It has unique advantages in the field of medical equipment by contrast with traditional processing technology. Especially, it plays an irreplaceable role in the surface processing of biological materials to improve the biocompatibility of materials. The latest application of laser microfabrication in the field of medical equipment manufacturing and processing in recent years is reviewed. The structure and surface manufacturing of vascular stents and bone stents, and the surface modification of biomaterials and antibacterial treatment are emphatically introduced. Finally, the limitations of the current laser micromachining technology are discussed, and the application and development of laser micromachining technology in medical equipment in the future are prospected.
-
Key words:
- laser micromachining /
- vascular stent /
- bone stent /
- biological materials /
- antibacterial
-
Overview
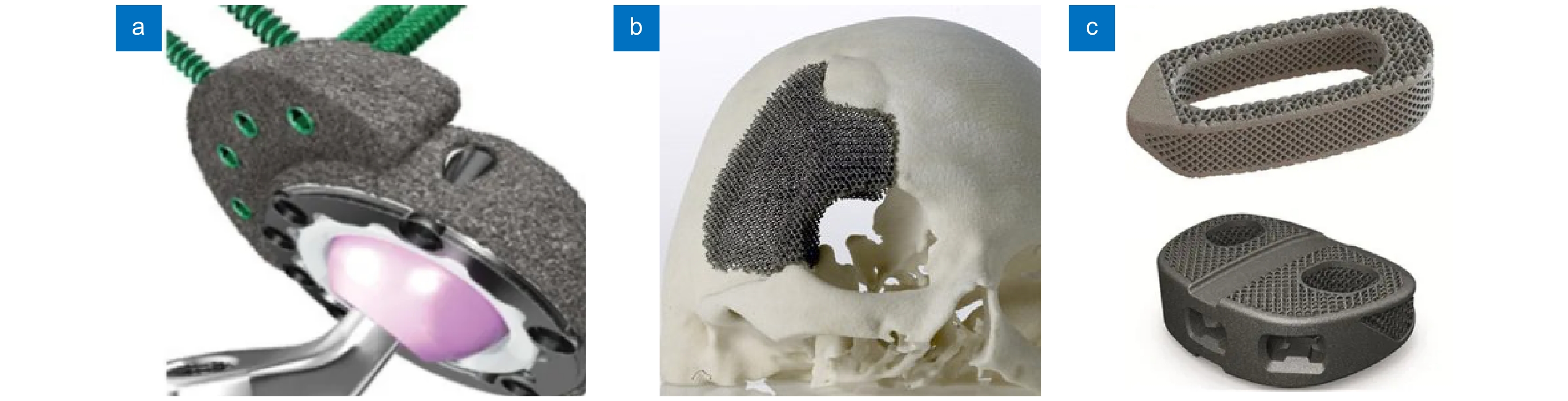
Overview: In recent years, the manufacturing industry has been developing in the direction of precision and high precision. In order to improve machining accuracy, scholars at home and abroad have carried out a lot of research on micro-machining. As a high-precision, green, and environment-friendly non-contact processing technology, laser processing has good flexibility and controllability. Because of its high accuracy, fast speed, small damage, and high power density, it has a broad application prospect in the biomedical field. Laser micro-processing technology endows biomaterials with new structures and functions, fully mobilizes the human body's self-repair ability, and realizes the permanent rehabilitation of damaged tissues or organs, which has become the development direction of contemporary biomedical science.
In order to systematically demonstrate the achievements of laser micromachining technology in the field of biomedicine, this paper analyzes the advantages of laser micromachining in the precision forming and surface modification of medical components from the manufacturing process and surface microstructure of medical devices, and summarizes the latest progress of laser micromachining technology in the manufacturing and processing of typical biomedical components. The influence of surface microstructures on the biocompatibility and antibacterial properties of medical components was explored. Furthermore, the achievements of laser micro-processing technology in the field of medical equipment manufacturing were systematically demonstrated.
Finally, the limitations of laser processing at present are summarized, and the application and development of laser micromachining technology in the field of medical equipment in the future are prospected. Although laser micro-processing technology can micro-process a new generation of implantable medical devices with extremely fine structure, making the commercial use of the next generation of implantable medical devices feasible, the development of laser micro-processing technology in the biomedical field is not mature enough, the production efficiency is low, and the work stability needs to be improved. For the laser micromachining process, a complete set of theories has not yet been formed to explain the physical nature of the interaction between the laser and material under the extreme conditions of ultra-fast, ultra-short, and ultra-strong, nor can the impact of laser micromachining on the material structure and physical and chemical properties be well evaluated. The next work still needs a lot of basic and regular research. At the same time, according to the characteristics of laser micromachining and the properties of the processed materials, it is also necessary to develop simulation analysis software to simulate the micromachining process and optimize the parameters of the laser micromachining process.
-

-
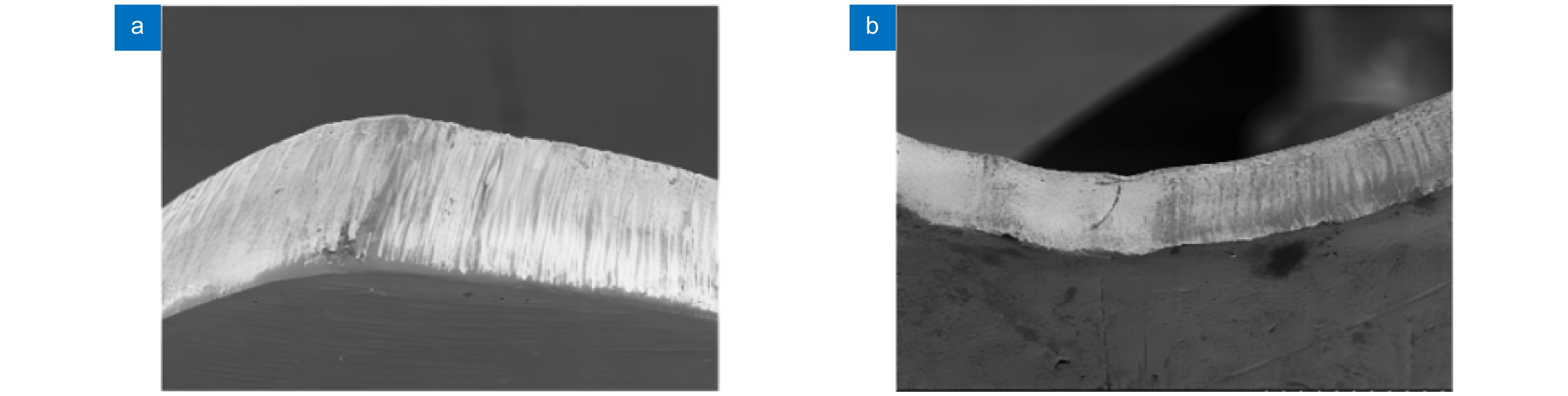
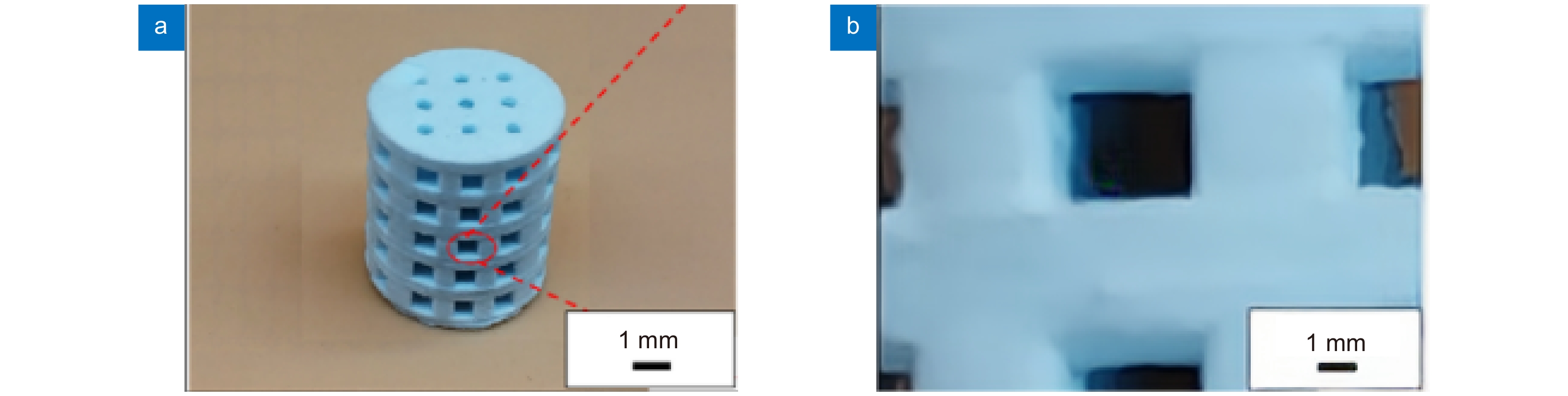
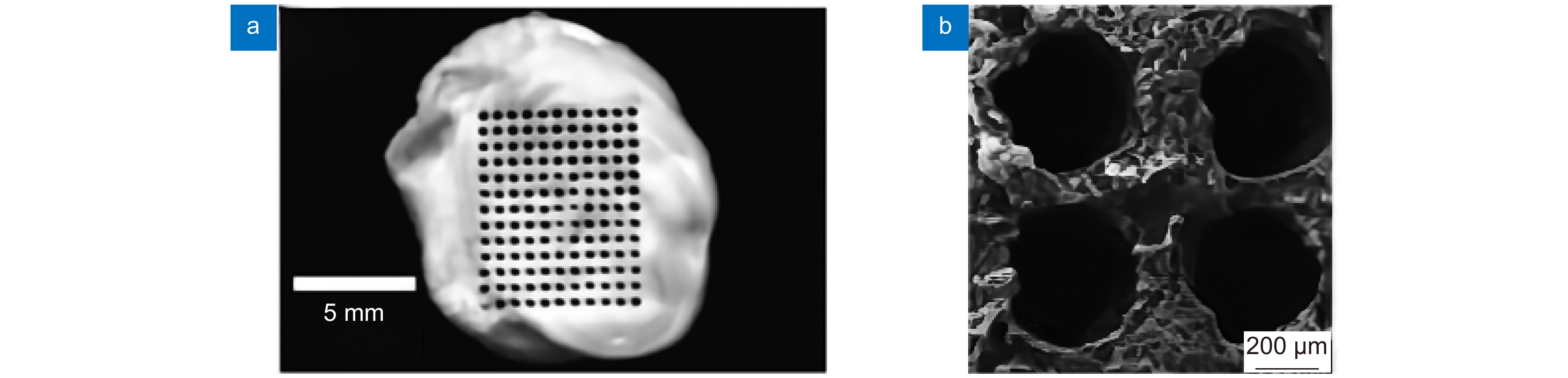
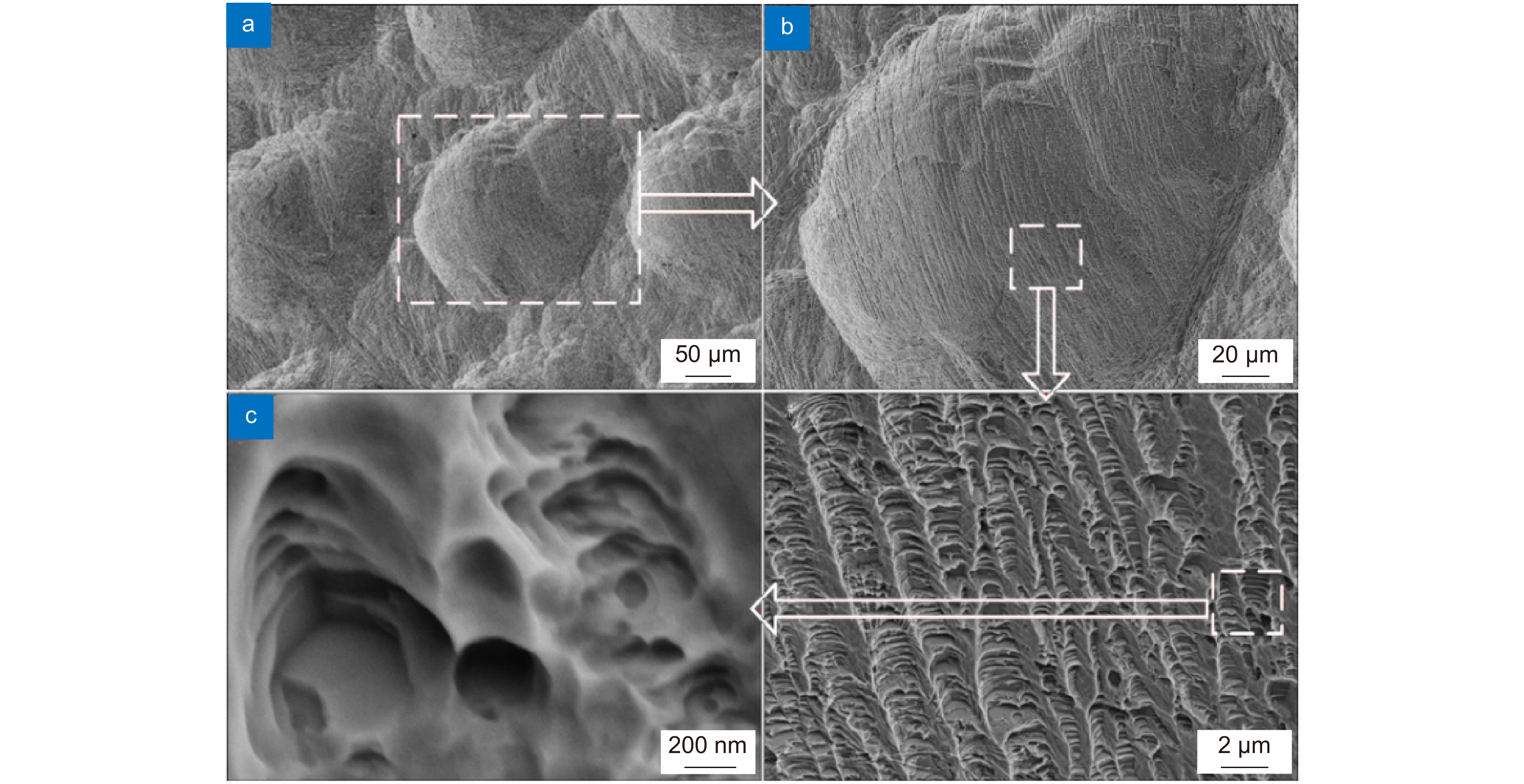
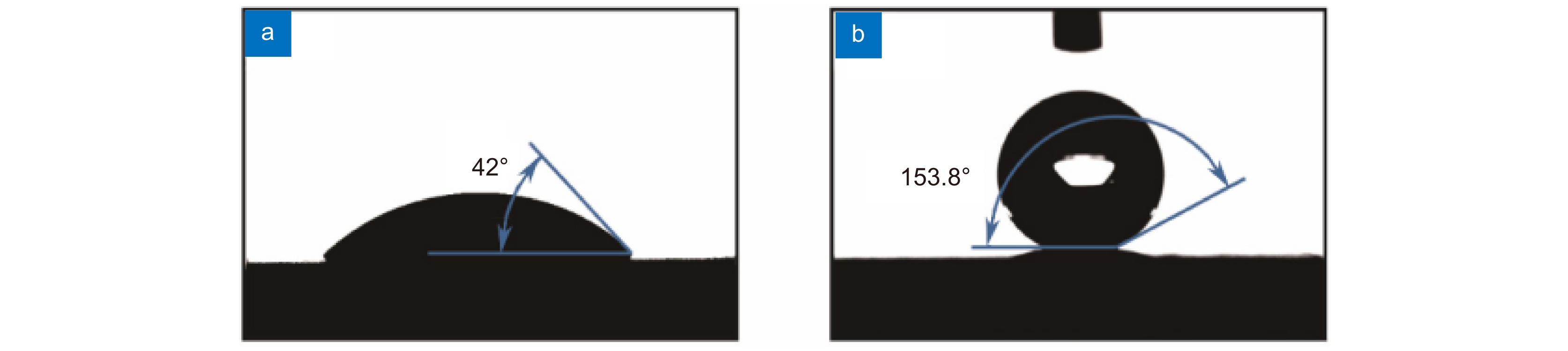
图 7 生物陶瓷支架激光制造[36] 。 (a) 激光沿着预定路径选择性地烧结β-TCP粉末;(b) 多孔β-TCP生物陶瓷支架的宏观形态;(c) 单个烧结路径的微观结构
Figure 7. Laser manufacturing of bioceramic stent[36]. (a) Selective laser sintering along a predetermined path β-TCP powder; (b) Porous β-Macro morphology of TCP bioceramic stent; (c) Microstructure of a single sintering path
表 1 医疗器械领域中激光加工成形技术
Table 1. Laser processing and forming technology in the field of medical devices
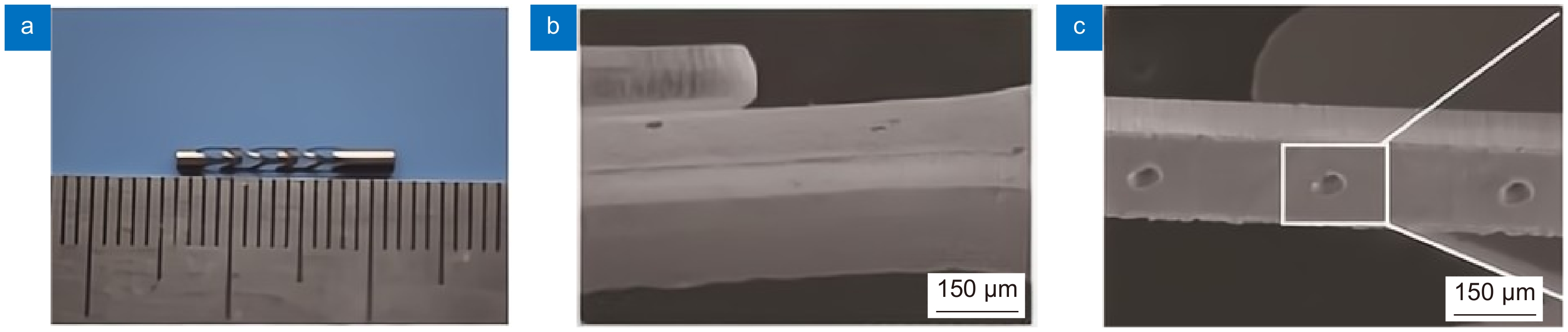
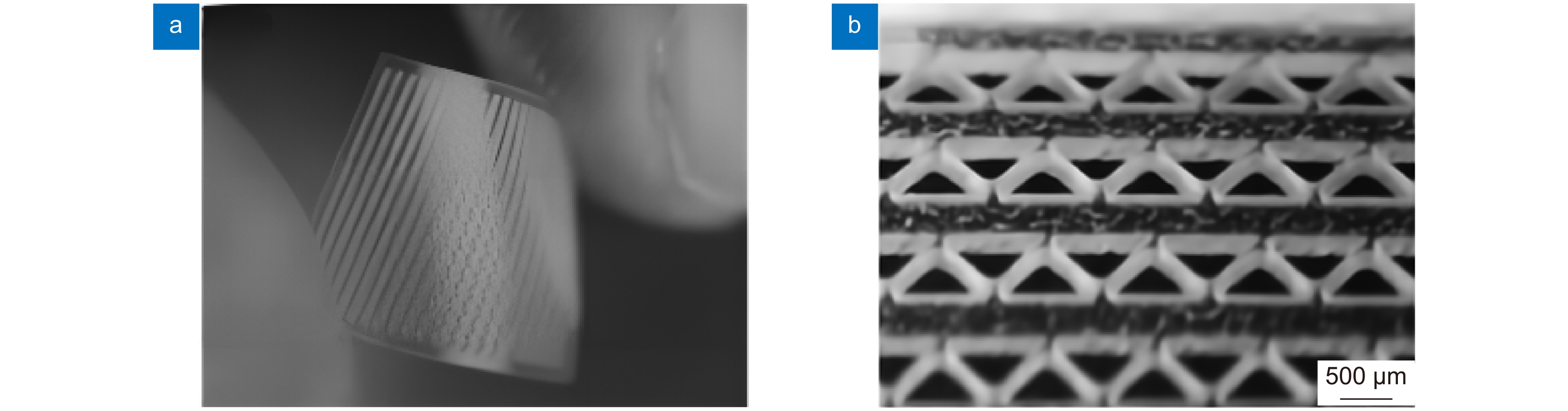
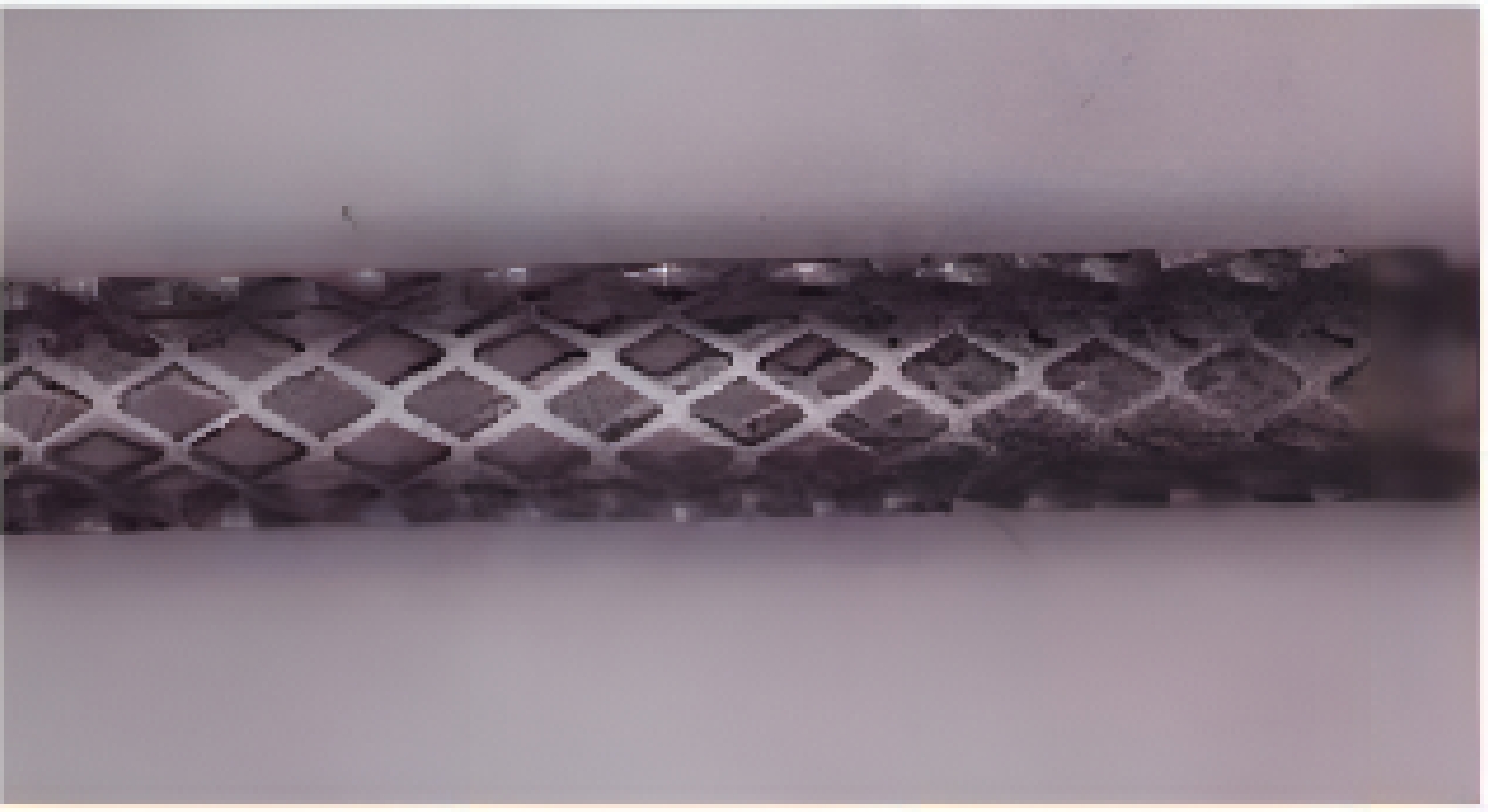
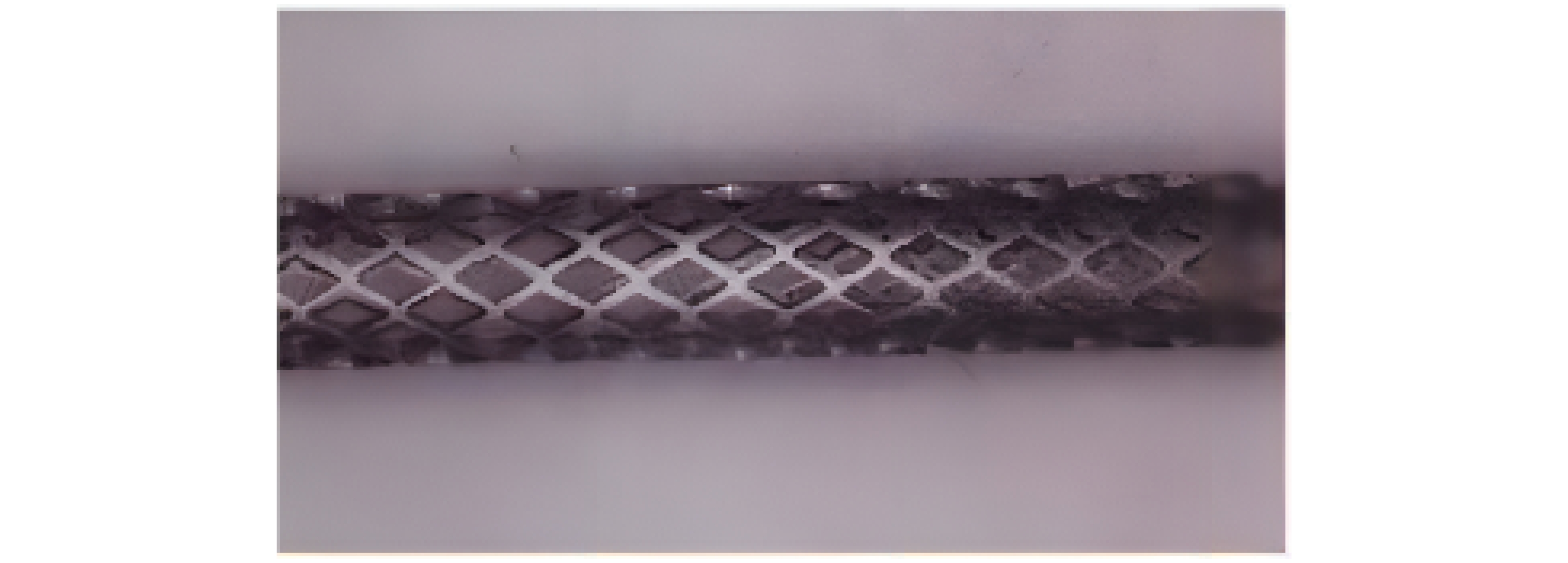
医疗器材 激光类型 加工材料 血管支架 飞秒激光,纳秒激光,皮秒激光,微秒激光 金属材料,可降解聚合物材料 骨支架 激光3D打印 金属材料,生物陶瓷等 表 2 血管支架激光加工材料及方法
Table 2. Materials and methods for laser processing of vascular stent
支架材料 激光加工方法 激光波长/nm 316L不锈钢 Nd:YAG激光,微秒激光,纳秒激光,飞秒激光 355~1064 钛及其合金 飞秒激光 1064 镁合金 飞秒激光-辅助气体 1064 高分子材料 飞秒激光-辅助气体,飞秒激光-辅助衬套 1030~1064 表 3 激光3D打印骨支架
Table 3. Laser 3D printing bone stent
骨支架材料 3D打印技术(微纳秒激光) 金属材料(钛,镁,不锈钢等) SLM 生物陶瓷 SL, SLA, SLS, DLP -
参考文献
[1] 杨涵, 于希辰, 吴一帆, 等. 飞秒激光在微加工领域的研究进展及其应用[J]. 应用激光, 2019, 39(2): 346−354. doi: 10.14128/j.cnki.al.20193902.346
Yang H, Yu X C, Wu Y F, et al. Research progress and application of femtosecond laser in microfabrication[J]. Appl Laser, 2019, 39(2): 346−354. doi: 10.14128/j.cnki.al.20193902.346
[2] Miller P R, Aggarwal R, Doraiswamy A, et al. Laser micromachining for biomedical applications[J]. JOM, 2009, 61(9): 35−40. doi: 10.1007/s11837-009-0130-7
[3] Ruddock I S, Bradley D J. Bandwidth-limited subpicosecond pulse generation in mode-locked cw dye lasers[J]. Appl Phys Lett, 1976, 29(5): 296−297. doi: 10.1063/1.89052
[4] Kamlage G, Bauer T, Ostendorf A, et al. Deep drilling of metals by femtosecond laser pulses[J]. Appl Phys A Mater Sci Process, 2003, 77(2): 307−310. doi: 10.1007/s00339-003-2120-x
[5] Gittard S D, Narayan R J. Laser direct writing of micro-and nano-scale medical devices[J]. Expert Rev Med Devices, 2010, 7(3): 343−356. doi: 10.1586/erd.10.14
[6] Chennupati R S, Trowers E A. Role of stents and laser therapy in biliary strictures[J]. Proc SPIE, 2001, 4244: 287−293. doi: 10.1117/12.427804
[7] Zhu Y L, Xie X Z, Huang Q P, et al. Research progress in laser processing of vascular stent[J]. Proc SPIE, 2018, 10827: 1082728. doi: 10.1117/12.2500022
[8] Meng H Y, Liao J H, Zhou Y H, et al. Laser micro-processing of cardiovascular stent with fiber laser cutting system[J]. Opt Laser Technol, 2009, 41(3): 300−302. doi: 10.1016/j.optlastec.2008.06.001
[9] Muhammad N, Whitehead D, Boor A, et al. Comparison of dry and wet fibre laser profile cutting of thin 316L stainless steel tubes for medical device applications[J]. J Mater Process Technol, 2010, 210(15): 2261−2267. doi: 10.1016/j.jmatprotec.2010.08.015
[10] Kathuria Y P. Laser microprocessing of metallic stent for medical therapy[J]. J Mater Process Technol, 2005, 170(3): 545−550. doi: 10.1016/j.jmatprotec.2005.05.041
[11] 张晋烨, 张海云, 李志永, 等. 激光参量对血管支架重铸层及热影响区的影响[J]. 激光技术, 2019, 43(4): 460−463. doi: 10.7510/jgjs.issn.1001-3806.2019.04.005
Zhang J Y, Zhang H Y, Li Z Y, et al. Effect of laser parameters on recasting layer and heat affected zone of cardiovascular stents[J]. Laser Technol, 2019, 43(4): 460−463. doi: 10.7510/jgjs.issn.1001-3806.2019.04.005
[12] 官邦贵, 廖健宏, 秦炎福, 等. 血管支架光纤激光切割技术[J]. 应用光学, 2009, 30(4): 678−682. doi: 10.3969/j.issn.1002-2082.2009.04.030
Guan B G, Liao J H, Qin Y F, et al. Cardiovascular stent cutting technique by fiber laser[J]. J Appl Opt, 2009, 30(4): 678−682. doi: 10.3969/j.issn.1002-2082.2009.04.030
[13] 李祥友, 曾晓雁. 激光精密切割不锈钢模板割缝质量控制[J]. 中国激光, 2002, 29(2): 176−180. doi: 10.3321/j.issn:0258-7025.2002.02.021
Li X Y, Zeng X Y. Kerf roughness and quality control of laser precision cutting[J]. Chin J Lasers, 2002, 29(2): 176−180. doi: 10.3321/j.issn:0258-7025.2002.02.021
[14] García-López E, Medrano-Tellez A G, Ibarra-Medina J R, et al. Experimental study of back wall dross and surface roughness in fiber Laser Microcutting of 316L miniature tubes[J]. Micromachines, 2018, 9(1): 4. doi: 10.3390/mi9010004
[15] Muhammad N, Whitehead D, Boor A, et al. Picosecond laser micromachining of nitinol and platinum-iridium alloy for coronary stent applications[J]. Appl Phys A Mater Sci Process, 2012, 106(3): 607−617. doi: 10.1007/s00339-011-6609-4
[16] 谢小柱, 朱裔良, 黄亚军, 等. 飞秒激光无碎屑加工不锈钢血管支架[J]. 机械工程学报, 2021, 57(5): 251−261. doi: 10.3901/JME.2021.05.251
Xie X Z, Zhu Y L, Huang Y J, et al. Debris-free femtosecond laser micromachining of stainless steel vascular stent[J]. J Mech Eng, 2021, 57(5): 251−261. doi: 10.3901/JME.2021.05.251
[17] Hung C H, Chang F Y. Curve micromachining on the edges of nitinol biliary stent by ultrashort pulses laser[J]. Opt Laser Technol, 2017, 90: 1−6. doi: 10.1016/j.optlastec.2016.10.018
[18] Demir A G, Previtali B, Colombo D, et al. Fiber laser micromachining of magnesium alloy tubes for biocompatible and Biodegradable cardiovascular stents[J]. Proc SPIE, 2012, 8237: 823730. doi: 10.1117/12.910131
[19] 张亚臣, 荣烨之. 医用聚乳酸的生物特性及其临床应用[J]. 上海医学, 2005, 28(1): 79−80. doi: 10.3969/j.issn.0253-9934.2005.01.032
Zhang Y C, Rong Y Z. Biological characteristics and clinical application of medical polylactic acid[J]. Shanghai Med J, 2005, 28(1): 79−80. doi: 10.3969/j.issn.0253-9934.2005.01.032
[20] Stolberg K, Friedel S, Kremser B, et al. IR and green femtosecond laser machining of heat sensitive materials for medical devices at micrometer scale[J]. Proc SPIE, 2014, 8968: 89680E. doi: 10.1117/12.2040665
[21] 位迪, 程萍, 陈向东, 等. 基于飞秒激光加工非金属血管支架的工艺研究[J]. 激光与光电子学进展, 2013, 50(9): 091403. doi: 10.3788/LOP50.091403
Wei D, Cheng P, Chen X D, et al. Study on femtosecond laser processing of nonmetal vascular stent[J]. Laser Optoelectron Prog, 2013, 50(9): 091403. doi: 10.3788/LOP50.091403
[22] 程萍, 位迪, 吴本科, 等. 可降解心脏支架的飞秒激光精密加工[J]. 光学 精密工程, 2014, 22(1): 63−68. doi: 10.3788/OPE.20142201.0063
Cheng P, Wei D, Wu B K, et al. Femtosecond laser precision machining of biodegradable heart Stent[J]. Opt Precis Eng, 2014, 22(1): 63−68. doi: 10.3788/OPE.20142201.0063
[23] Lv X C, Shen L, Wu Y Z, et al. Healing score of the Xinsorb scaffold in the treatment of de novo lesions: 6-month imaging outcomes[J]. Int J Cardiovasc Imaging, 2018, 34(7): 1009−1016. doi: 10.1007/s10554-018-1326-0
[24] Zhong F, Hu W, Zhu P N, et al. Piezoresistive design for electronic skin: from fundamental to emerging applications[J]. Opto-Electron Adv, 2022, 5(8): 210029. doi: 10.29026/oea.2022.210029
[25] Chiu Y W, Chen W P, Su C C, et al. The arrhythmogenic effect of self-assembling nanopeptide hydrogel scaffolds on neonatal mouse cardiomyocytes[J]. Nanomed Nanotechnol Biol Med, 2014, 10(5): 1065−1073. doi: 10.1016/j.nano.2014.01.005
[26] Jones J R, Ehrenfried L M, Hench L L. Optimising bioactive glass scaffolds for bone tissue engineering[J]. Biomaterials, 2006, 27(7): 964−973. doi: 10.1016/j.biomaterials.2005.07.017
[27] Van Bael S, Chai Y C, Truscello S, et al. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6Al4V bone scaffolds[J]. Acta Biomater, 2012, 8(7): 2824−2834. doi: 10.1016/j.actbio.2012.04.001
[28] 陈倩, 赵雪阳, 尤德强, 等. 3D打印纯钛骨支架表面掺银介孔生物活性玻璃涂层的性能研究[J]. 材料工程, 2022, 50(11): 34−45. doi: 10.11868/j.issn.1001-4381.2021.001215
Chen Q, Zhao X Y, You D Q, et al. Properties of Ag-doped mesoporous bioactive glass coatings on 3D printed pure titanium bone scaffolds[J]. J Mater Eng, 2022, 50(11): 34−45. doi: 10.11868/j.issn.1001-4381.2021.001215
[29] Parr W C H, Burnard J L, Singh T, et al. C3-C5 chordoma resection and reconstruction with a three-dimensional printed titanium patient-specific implant[J]. World Neurosurg, 2020, 136: 226−233. doi: 10.1016/j.wneu.2019.11.167
[30] Steele J R, Dekker T J, Federer A E, et al. Osteochondral lesions of the talus: current concepts in diagnosis and treatment[J]. Foot Ankle Orthop, 2018, 3(3). doi: 10.1177/2473011418779559
[31] Wang H, Su K X, Su L Z, et al. Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis[J]. Mater Sci Eng C Mater Biol Appl, 2019, 104: 109908. doi: 10.1016/j.msec.2019.109908
[32] Xin C, Yang L, Li J W, et al. Conical hollow microhelices with superior swimming capabilities for targeted cargo delivery[J]. Adv Mater, 2019, 31(25): 1808226. doi: 10.1002/adma.201808226
[33] 梁浩文, 王月, 陈小腾, 等. 3D打印生物陶瓷人工骨支架的研究进展[J]. 粉末冶金技术, 2022, 40(2): 100−109,117. doi: 10.19591/j.cnki.cn11-1974/tf.2021030040
Liang H W, Wang Y, Chen X T, et al. Progress of 3D printing bioceramic on artificial bone scaffolds[J]. Powder Metall Technol, 2022, 40(2): 100−109,117. doi: 10.19591/j.cnki.cn11-1974/tf.2021030040
[34] 李祥, 李涤尘, 王林, 等. 基于RP的骨组织工程支架构造及生物学特性分析[J]. 中国机械工程, 2005, 16(12): 1117−1120. doi: 10.3321/j.issn:1004-132X.2005.12.023
Li X, Li D C, Wang L, et al. Fabrication of bone tissue engineering scaffolds based on RP and analysis of biological properties[J]. China Mech Eng, 2005, 16(12): 1117−1120. doi: 10.3321/j.issn:1004-132X.2005.12.023
[35] 杨蒙蒙, 伍言龙, 许燕, 等. 生物玻璃陶瓷骨支架的光固化成形工艺与过固化研究[J]. 西安交通大学学报, 2022, 56(9): 151−159. doi: 10.7652/xjtuxb202209016
Yang M M, Wu Y L, Xu Y, et al. Forming process and overcure of bioglass ceramic bone scaffold by photocuring technology[J]. J Xi’an Jiaotong Univ, 2022, 56(9): 151−159. doi: 10.7652/xjtuxb202209016
[36] Shuai C J, Feng P, Zhang L Y, et al. Correlation between properties and microstructure of laser sintered porous β-tricalcium phosphate bone scaffolds[J]. Sci Technol Adv Mater, 2016, 14(5): 055002. doi: 10.1088/1468-6996/14/5/055002
[37] Feng C W, Zhang K Q, He R J, et al. Additive manufacturing of hydroxyapatite bioceramic scaffolds: dispersion, digital light processing, sintering, mechanical properties, and biocompatibility[J]. J Adv Ceram, 2020, 9(3): 360−373. doi: 10.1007/s40145-020-0375-8
[38] Lee U L, Lim J Y, Park S N, et al. A clinical trial to evaluate the efficacy and safety of 3D printed bioceramic implants for the reconstruction of zygomatic bone defects[J]. Materials, 2020, 13(20): 4515. doi: 10.3390/ma13204515
[39] Maroulakos M, Kamperos G, Tayebi L, et al. Applications of 3D printing on craniofacial bone repair: a systematic review[J]. J Dent, 2019, 80: 1−14. doi: 10.1016/j.jdent.2018.11.004
[40] Leong K F, Cheah C M, Chua C K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs[J]. Biomaterials, 2003, 24(13): 2363−2378. doi: 10.1016/S0142-9612(03)00030-9
[41] 王晶, 乌日开西·艾依提, 艾合买提江·玉素甫. 基于3D打印的胫骨骨折外固定生物力学研究[J]. 光电工程, 2021, 48(7): 200383. doi: 10.12086/oee.2021.200383
Wang J, Aiyiti W, Yusufu A. Biomechanical study on external fixation of tibial fractures based on 3D printing[J]. Opto-Electron Eng, 2021, 48(7): 200383. doi: 10.12086/oee.2021.200383
[42] 连伟龙, 连芩, 焦天, 等. 皮肤修复生物3D打印的研究进展与挑战[J]. 光电工程, 2021, 48(8): 210105. doi: 10.12086/oee.2021.210105
Lian W L, Lian Q, Jiao T, et al. The research progress and challenge of 3D bioprinting for skin repairing[J]. Opto-Electron Eng, 2021, 48(8): 210105. doi: 10.12086/oee.2021.210105
[43] Guo X Y, Tavakoli B, Kang H J, et al. Photoacoustic active ultrasound element for catheter tracking[J]. Proc SPIE, 2014, 8943: 89435m. doi: 10.1117/12.2041625
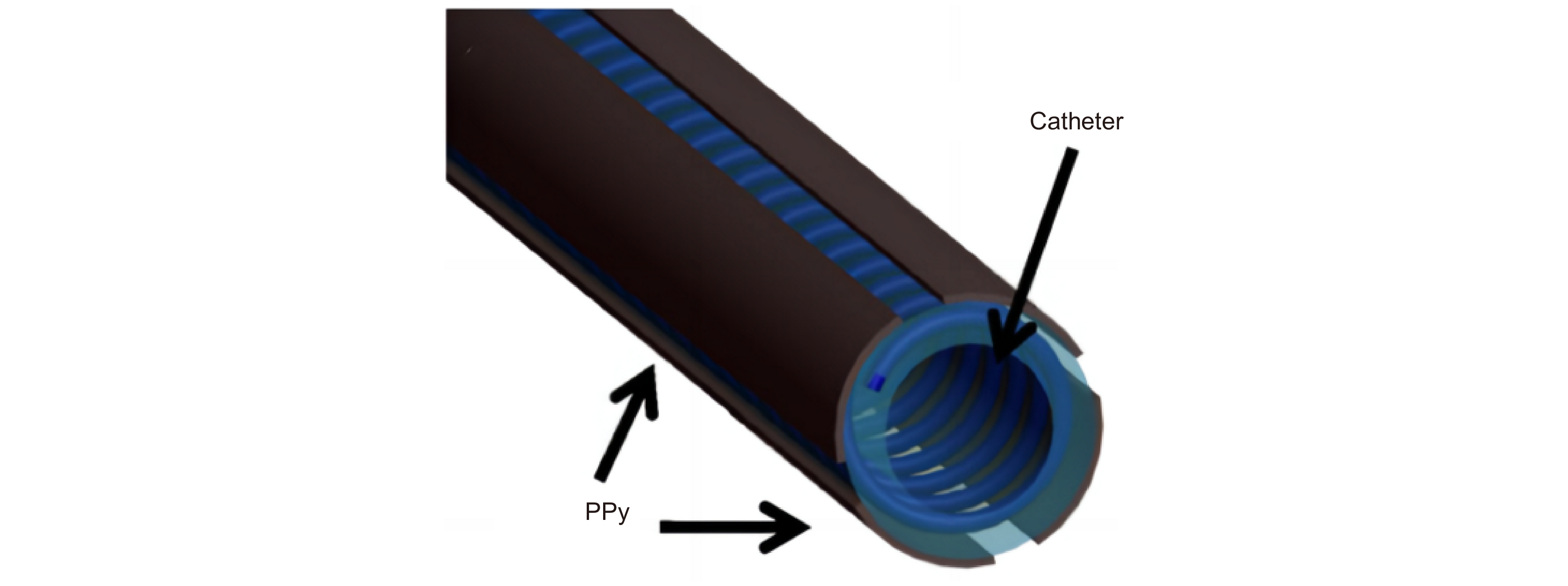
[44] Lee K K C, Munce N R, Shoa T, et al. Fabrication and characterization of laser-micromachined polypyrrole-based artificial muscle actuated catheters[J]. Sens Actuators A Phys, 2009, 153(2): 230−236. doi: 10.1016/j.sna.2009.05.005
[45] Zehbe R, Zehbe K. Strontium doped poly-ε-caprolactone composite scaffolds made by reactive foaming[J]. Mater Sci Eng C Mater Biol Appl, 2016, 67: 259−266. doi: 10.1016/j.msec.2016.05.045
[46] Kim M S, Son J G, Lee H J, et al. Highly porous 3D nanofibrous scaffolds processed with an electrospinning/laser process[J]. Curr Appl Phys, 2014, 14(1): 1−7. doi: 10.1016/j.cap.2013.10.008
[47] Wang J P, Valmikinathan C M, Liu W, et al. Spiral-structured, nanofibrous, 3D scaffolds for bone tissue engineering[J]. J Biomed Mater Res A, 2010, 93(2): 753−762. doi: 10.1002/jbm.a.32591
[48] 纪振冰, 万熠, 赵梓贺, 等. 生物医用钛植入体表面微纳结构与生物活性离子对生物相容性影响研究综述[J]. 中国表面工程, 2022, 35(4): 84−101.
Ji Z B, Wan Y, Zhao Z H, et al. Review on the influence of surface micro-nano structure and bioactive ions of biomedical titanium implants on biocompatibility[J]. China Surf Eng, 2022, 35(4): 84−101.
[49] Alves N M, Pashkuleva I, Reis R L, et al. Controlling cell behavior through the design of polymer surfaces[J]. Small, 2010, 6(20): 2208−2220. doi: 10.1002/smll.201000233
[50] Aebli N, Krebs J, Stich H, et al. In vivo comparison of the osseointegration of vacuum plasma sprayed titanium-and hydroxyapatite-coated implants[J]. J Biomed Mater Res A, 2003, 66(2): 356−363. doi: 10.1002/jbm.a.10508
[51] Liu X Y, Chu P K, Ding C X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications[J]. Mater Sci Eng R Rep, 2004, 47(3–4): 49−121. doi: 10.1016/j.mser.2004.11.001
[52] 王瑞, 周延民. 飞秒激光在钛合金表面改性中的应用及其对成骨细胞黏附和增殖的影响[J]. 吉林大学学报(医学版), 2013, 39(3): 453−457. doi: 10.7694/jldxyxb20130306
Wang R, Zhou Y M. Application of femto-second laser in titanium alloy surface modification and its influence in adhesion and proliferation of osteoblasts[J]. J Jilin Univ (Med Ed), 2013, 39(3): 453−457. doi: 10.7694/jldxyxb20130306
[53] Dumas V, Guignandon A, Vico L, et al. Femtosecond laser nano/micro patterning of titanium influences mesenchymal stem cell adhesion and commitment[J]. Biomed Mater, 2015, 10(5): 055002. doi: 10.1088/1748-6041/10/5/055002
[54] Cunha A, Zouani O F, Plawinski L, et al. Human mesenchymal stem cell behavior on femtosecond laser-textured Ti- 6Al -4V surfaces[J]. Nanomedicine, 2015, 10(5): 725−739. doi: 10.2217/nnm.15.19
[55] Zhang R, Wan Y, Ai X, et al. Preparation of micro-nanostructure on titanium implants and its bioactivity[J]. Trans Nonferrous Met Soc China, 2016, 26(4): 1019−1024. doi: 10.1016/S1003-6326(16)64217-6
[56] Allegrini Jr S, Yoshimoto M, Barbosa Salles M, et al. Biologic response to titanium implants with laser-treated surfaces[J]. Int J Oral Maxillofac Implants, 2014, 29(1): 63−70. doi: 10.11607/jomi.3213
[57] Ramazani S A A, Mousavi S A, Seyedjafari E, et al. Polycarbonate surface cell's adhesion examination after Nd: YAG laser irradiation[J]. Mater Sci Eng C Mater Biol Appl, 2009, 29(4): 1491−1497. doi: 10.1016/j.msec.2008.11.019
[58] 门博. 钛合金表面微纳结构设计制造及其生物活性研究[D]. 济南: 山东大学, 2015.
Men B. Design and manufacture of micro-nano-structured titanium alloy surface and its bioactivity[D]. Ji’nan: Shandong University, 2015.
[59] 虞宙, 张文杰, 胡俊. 皮秒激光对医用钛合金植入物表面微加工及生物相容性的研究[J]. 中国激光, 2017, 44(1): 0102014. doi: 10.3788/CJL201744.0102014
Yu Z, Zhang W J, Hu J. Micromachining of titanium alloy implant by picosecond laser surface texturing and alloy biocompatibility[J]. Chin J Lasers, 2017, 44(1): 0102014. doi: 10.3788/CJL201744.0102014
[60] 何婉盈, 姚鹏, 褚东凯, 等. 钛表面微凹凸织构的激光加工及其细胞黏附研究[J]. 中国激光, 2022, 49(10): 1002605. doi: 10.3788/CJL202249.1002605
He W Y, Yao P, Chu D K, et al. Fabrication and cell-adhesion evaluation of laser-ablated microprotrusion or microgroove on titanium[J]. Chin J Lasers, 2022, 49(10): 1002605. doi: 10.3788/CJL202249.1002605
[61] 杜策之, 王成勇. 医用金属材料抗菌表面制造[J]. 机电工程技术, 2020, 49(11): 16−19,111. doi: 10.3969/j.issn.1009-9492.2020.11.003
Du C Z, Wang C Y. Antibacterial surface processing on metallic biomaterials[J]. Mech Electr Eng Technol, 2020, 49(11): 16−19,111. doi: 10.3969/j.issn.1009-9492.2020.11.003
[62] Parvizi J, Wickstrom E, Zeiger A R, et al. Frank stinchfield award: titanium surface with biologic activity against infection[J]. Clin Orthop Relat Res, 2004, 429: 33−38. doi: 10.1097/01.blo.0000150116.65231.45
[63] Marambio-Jones C, Hoek E M V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment[J]. J Nanopart Res, 2010, 12(5): 1531−1551. doi: 10.1007/s11051-010-9900-y
[64] Zhao Y Z, Su Y L, Hou X Y, et al. Directional sliding of water: biomimetic snake scale surfaces[J]. Opto-Electron Adv, 2021, 4(4): 210008. doi: 10.29026/oea.2021.210008
[65] Zhao Y Z, Hong M H. Stainless steel anisotropic superhydrophobic surfaces fabrication with inclined cone array via laser ablation and post annealing treatment[J]. J Cent South Univ, 2022, 29(10): 3261−3269. doi: 10.1007/s11771-022-5145-z
[66] 泮怀海, 王卓, 范文中, 等. 飞秒激光诱导超疏水钛表面微纳结构[J]. 中国激光, 2016, 43(8): 0802002. doi: 10.3788/CJL201643.0802002
Pan H H, Wang Z, Fan W Z, et al. Superhydrophobic titanium surface micro/nanostructures induced by femtosecond laser[J]. Chin J Lasers, 2016, 43(8): 0802002. doi: 10.3788/CJL201643.0802002
[67] Dou X Q, Zhang D, Feng C L, et al. Bioinspired hierarchical surface structures with tunable wettability for regulating bacteria adhesion[J]. ACS Nano, 2015, 9(11): 10664−10672. doi: 10.1021/acsnano.5b04231
[68] Pan Q F, Cao Y, Xue W, et al. Picosecond laser-textured stainless steel Superhydrophobic surface with an antibacterial adhesion property[J]. Langmuir, 2019, 35(35): 11414−11421. doi: 10.1021/acs.langmuir.9b01333
[69] Žemaitis A, Mimidis A, Papadopoulos A, et al. Controlling the wettability of stainless steel from highly-hydrophilic to super-hydrophobic by femtosecond laser-induced ripples and nanospikes[J]. RSC Adv, 2020, 10(62): 37956−37961. doi: 10.1039/D0RA05665K
[70] 任乃飞, 宋佳佳, 李保家, 等. 飞秒激光刻蚀氧化锆表面微纳结构及其润湿与抗菌性能[J]. 表面技术, 2022, 51(9): 359−370. doi: 10.16490/j.cnki.issn.1001-3660.2022.09.038
Ren N F, Song J J, Li B J, et al. Micro-nano structures, wettability and antibacterial property on zirconia surfaces by femtosecond laser etching[J]. Surf Technol, 2022, 51(9): 359−370. doi: 10.16490/j.cnki.issn.1001-3660.2022.09.038
-
访问统计


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: