Rapid preparation of high-precision hydrogel micropatterns and its induction of cell behavior
-
摘要:
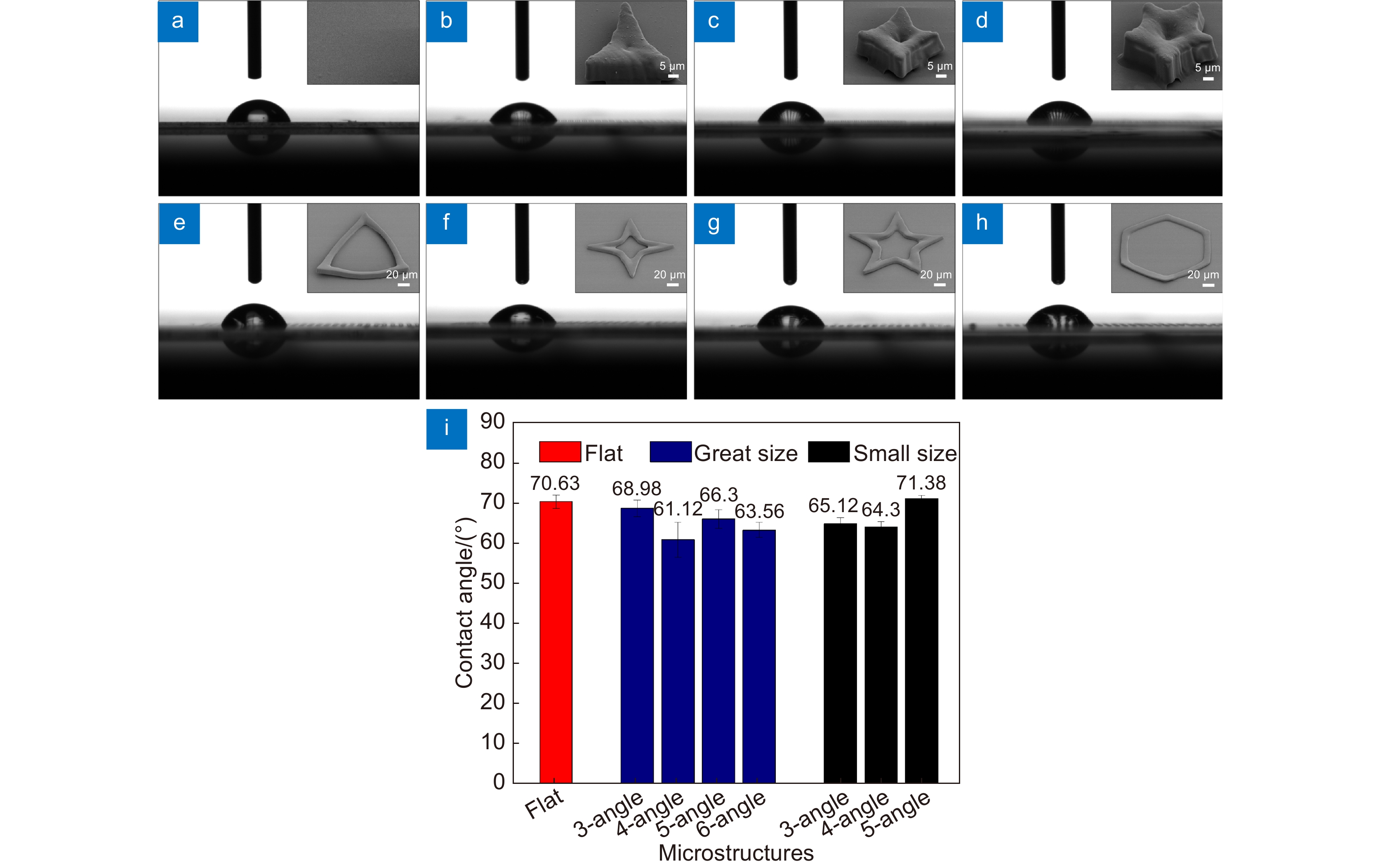
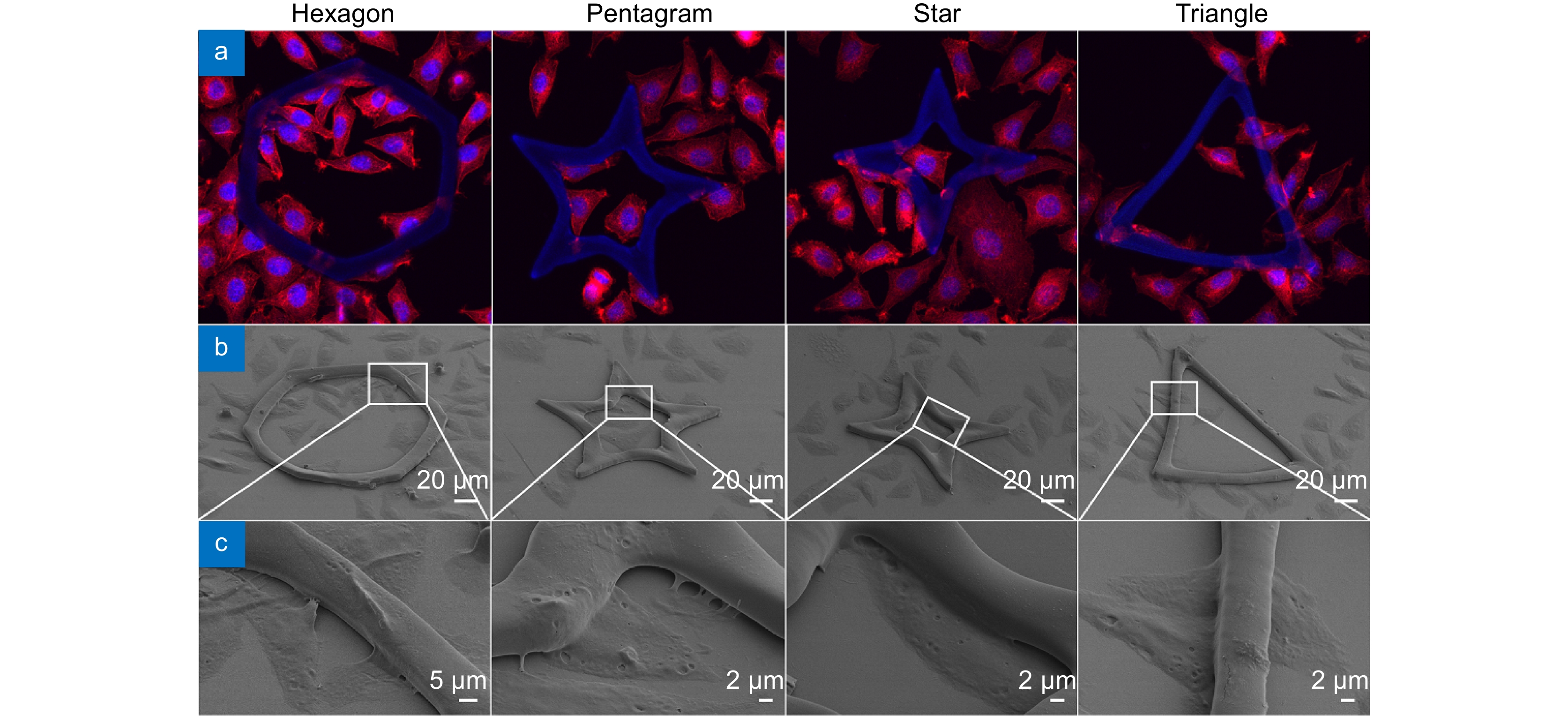
为探索不同尺寸的水凝胶微图案对细胞的诱导调控作用,本文采用飞秒激光无掩模投影光刻技术,将水凝胶前驱体溶液制备成所设计的图案,同时结合大面积拼接,获得了具有大面积、不同尺寸的多边形和多角星微结构。详细研究了微结构的最佳加工条件及其浸润性。带有微结构的基底与成纤维细胞共培养的实验结果表明,微结构的空间限位作用会改变细胞形貌,从而能够对细胞的生长行为进行有效地调控。尤其在小尺寸的多边形和多角星微结构上,细胞核会落入微结构的中心陷窝,细胞骨架则不断铺展分布,并逐渐与微结构形貌趋于一致。该研究证实了微结构图案单元尺寸对诱导细胞行为功能至关重要,将为利用生物相容性水凝胶微结构进行体外细胞研究提供新技术与新方法。
Abstract:In order to investigate the regulation effect of hydrogel micropatterns of different sizes on cell behaviors, large-area polygons and polygonal microstructures of different sizes were achieved by a femtosecond laser maskless optical projection lithography technique, in combination with a large-area splicing method. The optimum processing conditions and the wettability of the microstructure are studied in detail. The coculture experimental results of fibroblasts with patterned substrates showed that the spatial confinement in the microstructure can change the cell morphology and effectively regulate the cell growth behaviors. In particular, in the small polygonal and polygonal star microstructure, the nucleus tends to fall into the concave lacunae of the microstructure, and the spreading of cytoskeleton gradually tends to be consistent with the morphology of microstructures. This study indicates that the size of microstructural pattern units is very important for inducing cell behavior and function, which would provide a new technology and new method for cell research in vitro by using biocompatible hydrogel microstructures.
-
Key words:
- maskless optical projection lithography /
- hydrogel /
- micropattern /
- cell behavior
-
Overview: Previous researches have indicated that pattern surfaces usually exhibit contact induction effects on cells. The microstructure can effectively regulate the adhesion and proliferation of cells. Cell morphology and cellular function are inseparable. For example, stem cells have a large degree of differentiation of different lineage depending on the adhesion form of the cell. Nanostructures affect cell adhesion behavior at the molecular level. Nanowires have been used to stimulate and record individual neuronal activities, and nanopores can be used for single-cell detection. Therefore, it will bring significant contributions to the fields of organizational engineering to create a variety of scales, different topological substrate in vitro to investigate cell-material, cell-environmental interaction mechanism.
Hydrogels are commonly used in tissue engineering since they have a similar component to the extracellular matrix and have advantages such as good biocompatibility, non-toxic and degradability. A large number of bionic cell scaffolds have been prepared by techniques such as microfluidic technology, 3D printing, soft printing, self-assembly, ultraviolet photolithography etc. This provides important guidance for cancer transfer, wound healing and inflammatory treatment. However, the sizes of these microstructures are usually large with a relatively low structure resolution, accounting for the limitation of hydrogel properties and manufacturing methods. Thus, it is still a challenge to rapidly fabricate large-area hydrogel micro/nano-structures with complex and arbitrary patterns.
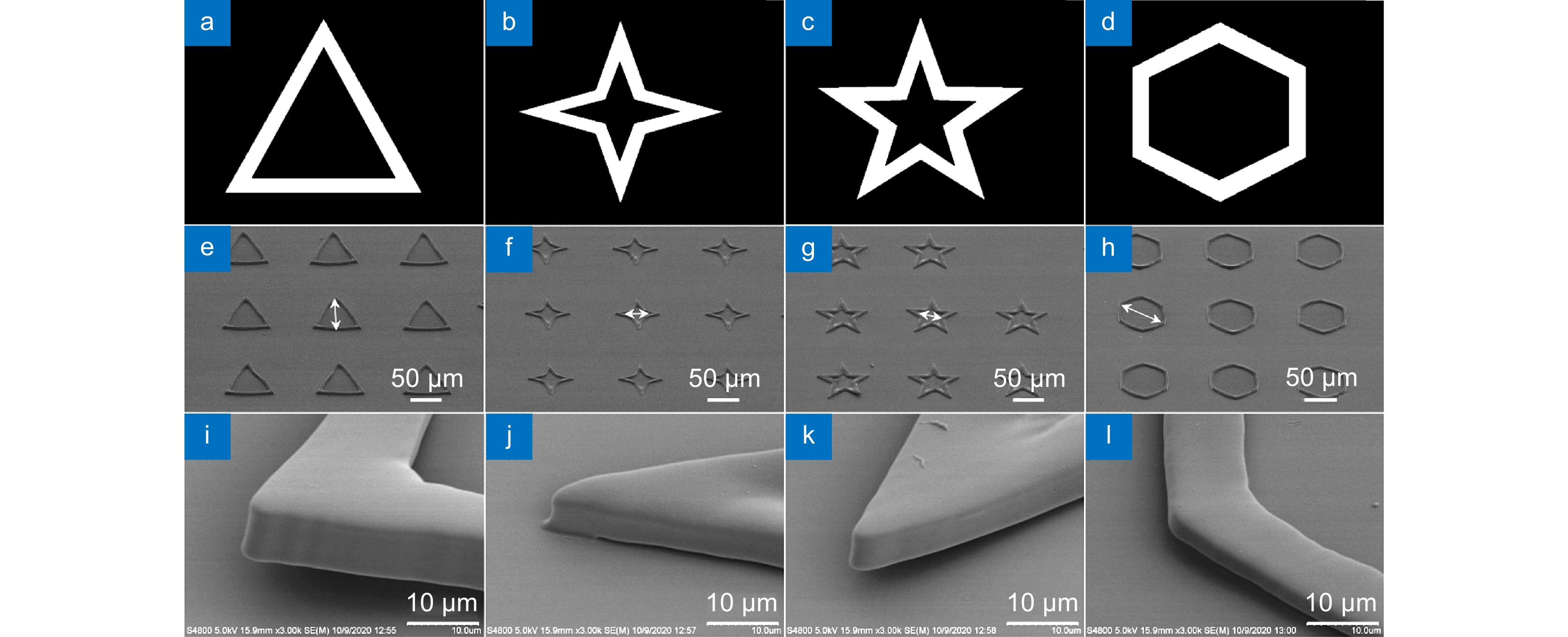
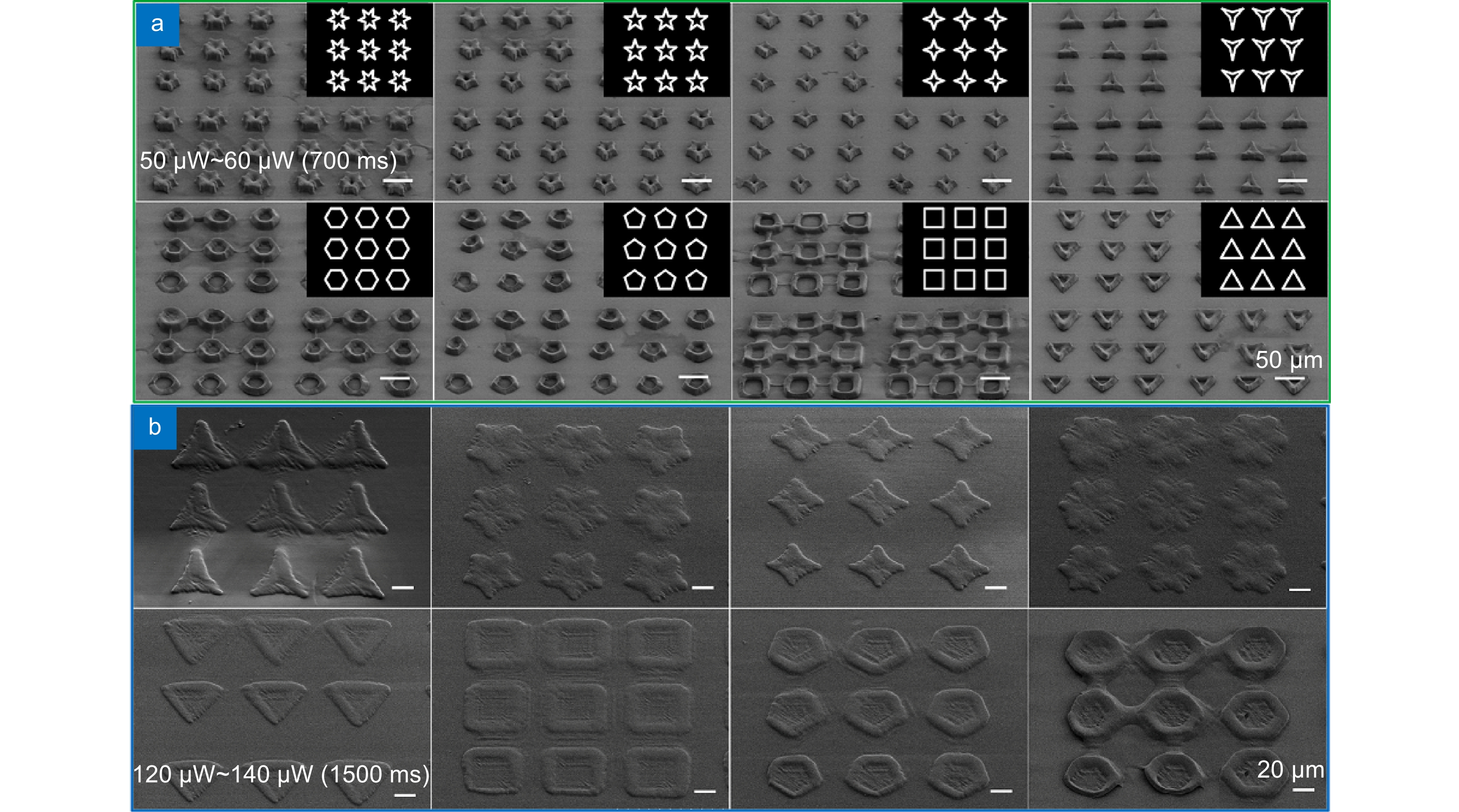
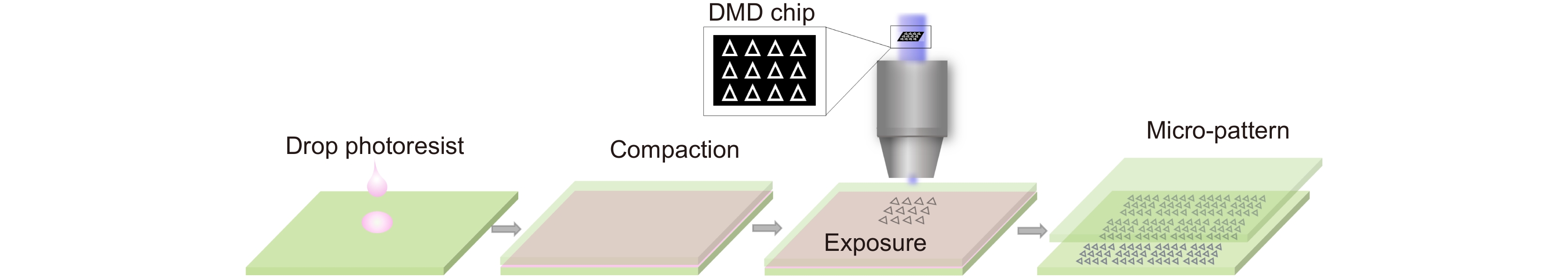
In this paper, we have prepared the hydrogel by using PEGDA, PE-3A and the mixing photoinitiators (Irgure 369 and Benzil) with the weight ratios of 39.2: 59.2: 0.8: 0.8. A home-made maskless optical projection photolithography system is used to fabricate hydrogel patterns. A femtosecond laser, as the light source and high numerical aperture objective lens are used to improve structural resolution, and a splicing method is used to obtain large-area structures with high processing efficiency. Large-area polygons and polygonal stars with cavities in the center of itself are fabricated. First, the optimum processing conditions and the wettability of substrate with different patterns are studied. Then, Fibroblasts L929 are cultured on all kinds of pre-fabricated patterns. The cell behavior in micropattern with large cavity is similar to those on 2D flat substrates. Only the skeleton of cells close to the microstructure will produce deformation and interaction. For micropatterns with small sizes of cavities, the length of cells is significantly reduced because of the small space limit. In particular, the cell skeleton on the small-sized micropatterns exhibits a consistent distribution of the topography. The nuclear would fall into the center depression likes “bone trap” due to gravity. This study indicates that the size of microstructural pattern units is very important for inducing cell behavior and function, which would provide a novel method to prepare hydrogel micropatterns to study cell behavior in the field of organizational engineering.
-

-
-
[1] Yu S Z, Liu D, Wang T Y, et al. Micropatterning of polymer substrates for cell culture[J]. J Biomed Mater Res Part B Appl Biomater, 2021, 109(10): 1525−1533. doi: 10.1002/jbm.b.34811
[2] Ren K F, Hu M, Zhang H, et al. Layer-by-layer assembly as a robust method to construct extracellular matrix mimic surfaces to modulate cell behavior[J]. Prog Polym Sci, 2019, 92: 1−34. doi: 10.1016/j.progpolymsci.2019.02.004
[3] Yang J Y, Ting Y C, Lai J Y, et al. Quantitative analysis of osteoblast-like cells (MG63) morphology on nanogrooved substrata with various groove and ridge dimensions[J]. J Biomed Mater Res Part A, 2009, 90A(3): 629−640. doi: 10.1002/jbm.a.32130
[4] Zhou X T, Hu J, Li J J, et al. Patterning of two-level topographic cues for observation of competitive guidance of cell alignment[J]. ACS Appl Mater Interfaces, 2012, 4(8): 3888−3892. doi: 10.1021/am301237j
[5] Szabó Á, Liliom H, Fekete Z, et al. SU-8 microstructures alter the attachment and growth of glial cells in vitro[J]. Mater Today Commun, 2021, 27: 102336. doi: 10.1016/j.mtcomm.2021.102336
[6] Jahed Z, Molladavoodi S, Seo B B, et al. Cell responses to metallic nanostructure arrays with complex geometries[J]. Biomaterials, 2014, 35(34): 9363−9371. doi: 10.1016/j.biomaterials.2014.07.022
[7] Nomura S, Kojima H, Ohyabu Y, et al. Nanopillar sheets as a new type of cell culture dish: detailed study of HeLa cells cultured on nanopillar sheets[J]. J Artif Organs, 2006, 9(2): 90−96. doi: 10.1007/s10047-006-0329-0
[8] Kuo C W, Shiu J Y, Chien F C, et al. Polymeric nanopillar arrays for cell traction force measurements[J]. Electrophoresis, 2010, 31(18): 3152−3158. doi: 10.1002/elps.201000212
[9] Chang R, Yan Q F, Kingshot P, et al. Harnessing the perinuclear actin cap (pnAC) to influence nanocarrier trafficking and gene transfection efficiency in skeletal myoblasts using nanopillars[J]. Acta Biomater, 2020, 111: 221−231. doi: 10.1016/j.actbio.2020.05.015
[10] Robinson J T, Jorgolli M, Shalek A K, et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits[J]. Nat Nanotechnol, 2012, 7(3): 180−184. doi: 10.1038/nnano.2011.249
[11] Cheng Y J, Zhu S Y, Pang S W. Directing osteoblastic cell migration on arrays of nanopillars and nanoholes with different aspect ratios[J]. Lab Chip, 2021, 21(11): 2206−2216. doi: 10.1039/D1LC00104C
[12] Yang J M, Olanrele O S, Zhang X, et al. Fabrication of hydrogel materials for biomedical applications[M]//Chun H J, Park K, Kim C H, et al. Novel Biomaterials for Regenerative Medicine. Singapore: Springer, 2018: 197–224.
[13] Yu H, Liu J, Zhao Y Y, et al. Biocompatible three-dimensional hydrogel cell scaffold fabricated by sodium hyaluronate and chitosan assisted two-photon polymerization[J]. ACS Appl Bio Mater, 2019, 2(7): 3077−3083. doi: 10.1021/acsabm.9b00384
[14] Zheng Y C, Zhao Y Y, Zheng M L, et al. Cucurbit[7]uril-carbazole two-photon photoinitiators for the fabrication of biocompatible three-dimensional hydrogel scaffolds by laser direct writing in aqueous solution[J]. ACS Appl Mater Interfaces, 2019, 11(2): 1782−1789. doi: 10.1021/acsami.8b15011
[15] Wei S X, Liu J, Zhao Y Y, et al. Protein-based 3D microstructures with controllable morphology and pH-responsive properties[J]. ACS Appl Mater Interfaces, 2017, 9(48): 42247−42257. doi: 10.1021/acsami.7b14915
[16] Zhang W C, Zheng M L, Liu J, et al. Modulation of cell behavior by 3D biocompatible hydrogel microscaffolds with precise configuration[J]. Nanomaterials, 2021, 11(9): 2325. doi: 10.3390/nano11092325
[17] Gao W, Chao H, Zheng Y C, et al. Ionic carbazole-based water-soluble two-photon photoinitiator and the fabrication of biocompatible 3D hydrogel scaffold[J]. ACS Appl Mater Interfaces, 2021, 13(24): 27796−27805. doi: 10.1021/acsami.1c02227
[18] Zheng C L, Jin F, Zhao Y Y, et al. Light-driven micron-scale 3D hydrogel actuator produced by two-photon polymerization microfabrication[J]. Sens Actuators B Chem, 2020, 304: 127345. doi: 10.1016/j.snb.2019.127345
[19] Feng L, Liang S J, Zhou Y Y, et al. Three-dimensional printing of hydrogel scaffolds with hierarchical structure for scalable stem cell culture[J]. ACS Biomater Sci Eng, 2020, 6(5): 2995−3004. doi: 10.1021/acsbiomaterials.9b01825
[20] González-Henríquez C M, Rodriguez-Umanzor F E, Sarabia-Vallejos M A, et al. Innovative procedure for precise deposition of wrinkled hydrogel films using direct inkjet printing[J]. Mater Des, 2020, 194: 108959. doi: 10.1016/j.matdes.2020.108959
[21] Kim D H, Kim P, Song I, et al. Guided three-dimensional growth of functional cardiomyocytes on polyethylene glycol nanostructures[J]. Langmuir, 2006, 22(12): 5419−5426. doi: 10.1021/la060283u
[22] Erkoc-Biradli F Z, Ozgun A, Öztürk-Öncel Ö, et al. Bioinspired hydrogel surfaces to augment corneal endothelial cell monolayer formation[J]. J Tissue Eng Regen Med, 2021, 15(3): 244−255. doi: 10.1002/term.3173
[23] Li G C, Li S J, Zhang L L, et al. Construction of biofunctionalized anisotropic hydrogel micropatterns and their effect on Schwann cell behavior in peripheral nerve regeneration[J]. ACS Appl Mater Interfaces, 2019, 11(41): 37397−37410. doi: 10.1021/acsami.9b08510
[24] Kim G, Jung Y, Cho K, et al. Thermoresponsive poly(N-isopropylacrylamide) hydrogel substrates micropatterned with poly(ethylene glycol) hydrogel for adipose mesenchymal stem cell spheroid formation and retrieval[J]. Mater Sci Eng C, 2020, 115: 111128. doi: 10.1016/j.msec.2020.111128
[25] Noh S, Gong H Y, Lee H J, et al. Electrically conductive micropatterned polyaniline-poly(ethylene glycol) composite hydrogel[J]. Materials, 2021, 14(2): 308. doi: 10.3390/ma14020308
[26] Noh M, Choi Y H, An Y H, et al. Magnetic nanoparticle-embedded hydrogel sheet with a groove pattern for wound healing application[J]. ACS Biomater Sci Eng, 2019, 5(8): 3909−3921. doi: 10.1021/acsbiomaterials.8b01307
[27] Diez M, Schulte V A, Stefanoni F, et al. Molding micropatterns of elasticity on PEG-based hydrogels to control cell adhesion and migration[J]. Adv Eng Mater, 2011, 13(10): B395−B404. doi: 10.1002/adem.201080122
[28] Ha M, Athirasala A, Tahayeri A, et al. Micropatterned hydrogels and cell alignment enhance the odontogenic potential of stem cells from apical papilla in-vitro[J]. Dent Mater, 2020, 36(1): 88−96. doi: 10.1016/j.dental.2019.10.013
[29] Rahmouni S, Lindner A, Rechenmacher F, et al. Hydrogel micropillars with integrin selective peptidomimetic functionalized nanopatterned tops: a new tool for the measurement of cell traction forces transmitted through αvβ3-or α5β1-integrins[J]. Adv Mater, 2013, 25(41): 5869−5874. doi: 10.1002/adma.201301338
[30] Morishima K, Tanaka Y, Ebara M, et al. Demonstration of a bio-microactuator powered by cultured cardiomyocytes coupled to hydrogel micropillars[J]. Sens Actuators B Chem, 2006, 119(1): 345−350. doi: 10.1016/j.snb.2005.11.063
[31] Lee J Y, Shah S S, Yan J, et al. Integrating sensing hydrogel microstructures into micropatterned hepatocellular cocultures[J]. Langmuir, 2009, 25(6): 3880−3886. doi: 10.1021/la803635r
[32] Lee H J, Kim D N, Park S, et al. Micropatterning of a nanoporous alumina membrane with poly(ethylene glycol) hydrogel to create cellular micropatterns on nanotopographic substrates[J]. Acta Biomater, 2011, 7(3): 1281−1289. doi: 10.1016/j.actbio.2010.11.006
[33] Peng R, Yao X, Ding J D. Effect of cell anisotropy on differentiation of stem cells on micropatterned surfaces through the controlled single cell adhesion[J]. Biomaterials, 2011, 32(32): 8048−8057. doi: 10.1016/j.biomaterials.2011.07.035
[34] Chen S J, Zhang L G, Zhao Y, et al. A perforated microhole-based microfluidic device for improving sprouting angiogenesis in vitro[J]. Biomicrofluidics, 2017, 11(5): 054111. doi: 10.1063/1.4994599
[35] Emami M M, Barazandeh F, Yaghmaie F. Scanning-projection based stereolithography: method and structure[J]. Sens Actuators A Phys, 2014, 218: 116−124. doi: 10.1016/j.sna.2014.08.002
[36] Liu Y H, Zhao Y Y, Jin F, et al. λ/12 super resolution achieved in maskless optical projection nanolithography for efficient cross-scale patterning[J]. Nano Lett, 2021, 21(9): 3915−3921. doi: 10.1021/acs.nanolett.1c00559
[37] 高文, 郑美玲, 金峰, 等. 飞秒激光快速制备大面积二维微纳结构[J]. 激光与光电子学进展, 2020, 57(11): 111421. doi: 10.3788/LOP57.111421
Gao W, Zheng M L, Jin F, et al. Fast fabrication of large-area two-dimensional micro/nanostructure by femtosecond laser[J]. Laser Optoelect Prog, 2020, 57(11): 111421. doi: 10.3788/LOP57.111421
[38] Wang Y W, Wu Q, Chen G Q. Reduced mouse fibroblast cell growth by increased hydrophilicity of microbial polyhydroxyalkanoates via hyaluronan coating[J]. Biomaterials, 2003, 24(25): 4621−4629. doi: 10.1016/S0142-9612(03)00356-9
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: