-
摘要:
皮肤作为人体最大的器官,有着屏障功能、免疫应答、防止水分流失和排泄废物等重要作用。大面积的严重皮肤损伤患者会由于缺乏充足的可移植皮肤而死亡。生物3D打印技术的发展为可移植皮肤的制造提供了解决办法。生物3D打印技术可以定制化制造功能性的皮肤替代品,有望解决移植皮肤短缺的困难。本文简述了皮肤创伤修复原理,比较了用于皮肤修复的生物材料墨水、细胞和主要的生物3D打印技术,分析了光电技术在3D打印皮肤上的应用潜力,并总结了生物3D打印技术在皮肤修复应用中所面临的挑战和未来的发展方向,提出了光电技术在生物3D打印中的应用需求。
Abstract:The skin is the largest organ of the human body, which plays an important role in barrier function, immune response, preventing water loss and excreting waste. Patients with large-scale severe skin injuries will die due to lack of adequate skin grafts. The development of 3D bioprinting technology provides a solution for the manufacture of transplantable skin. Firstly, the principles of skin wound repairing are described. Secondly, the bioinks, cells and main 3D bioprinting technologies used in skin wound repairing are compared. Then, the opto-electronic technologies involved are analyzed, and the challenges and future development of 3D bioprinting in the application of skin repair are summarized. Finally, the application requirements of opto-electronic technology in 3D bioprinting are proposed.
-
Key words:
- 3D bioprinting /
- skin /
- bioink /
- cell /
- opto-electronic technology
-

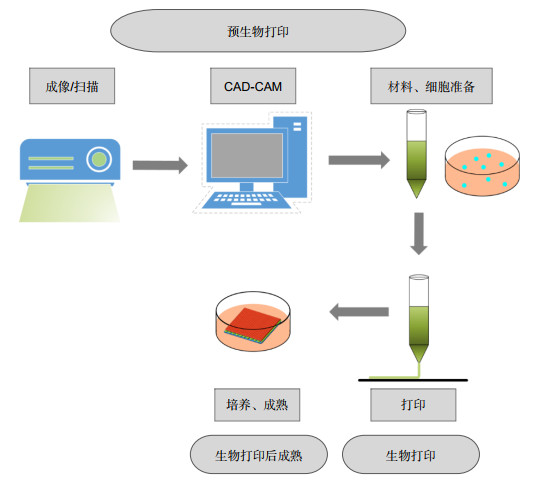
Overview: The skin is the first line of defense against external stimuli. Therefore, the skin is most vulnerable to injury, and serious skin injury may be life-threatening, so repairing damaged skin is of great significance. Because 3D bioprinting is able to accurately place a variety of different types of cells, even stem cells and appendages, and can repeatably create skin substitutes to replace the injured or damaged parts of the skin, making it similar to the skin appearance and function, 3D bioprinting makes up for the shortcomings of conventional skin wound repairing treatment, and is currently one of the most likely manufacturing methods to develop skin substitutes.
In order to improve the accuracy of printed skin, the degree of adaptation to the wound, and the effect of skin wound repairing, more and more opto-electronic technologies have been applied in 3D bioprinting. Piezoelectric and laser pulse technology can be used in the nozzle to obtain droplets with more uniform cell distribution and droplet diameters that are more suitable for inkjet printing. Digital mask projection technology uses digital micromirror to control the mask to print photosensitive materials to obtain high-resolution customized patterns. Laser-induced forward transfer technology adjusts the energy, spot size, and duration of the pulsed laser beam to cover the laser energy absorbing layer with bioink containing cells. Near-infrared fluorescence technology is used to monitor the printing process in real time, so as to adjust and plan the printing path in real time and obtain the skin with higher degree of compatibility with the defective skin.
In this article, firstly, the skin tissue structures and the principles of skin wound repairing are described. Secondly, the bioinks, cells and main 3D bioprinting technologies used in skin wound repairing are compared. Then, the opto-electronic technologies involved are analyzed. Finally, the application requirements of opto-electronic technology in 3D bioprinting are proposed.
-

-
表 1 天然聚合物类生物材料的特点及应用
Table 1. The characteristics and applications of natural polymer biomaterials
生物材料 特点 应用 纤维蛋白 天然存在于血液中,在凝血酶的作用下变成水凝胶状,在早期愈合中模拟了临时的ECM,所以可以促进血液凝结和伤口愈合[19]。 使用稀释的血浆来源的纤维蛋白在transwell系统中共培养角质形成细胞和成纤维细胞可增加胶原蛋白的表达,同时减少其迁移[20];创建了一个由成纤维细胞和含有纤维蛋白原的人血浆组成的真皮隔室,制造出了双层皮肤替代品[21]。 胶原蛋白 具有螺旋结构的三条多肽链组成,在37 ℃下具有一定的结构稳定性,而且其含有精氨酸-甘氨酸-天冬氨酸(RGD)序列的原纤维结构为细胞提供了结构和生物学支持,可促进细胞附着和增殖[22]。 制备了含有成纤维细胞和角质形成细胞的胶原蛋白-糖胺聚糖支架,用于烧伤创面治疗[23];采用了生物相容性交联方法或设计良好的胶原蛋白基质内的聚合物网格,通过pH值或温度控制或两者共同引发的其凝胶化[24-25]。 明胶 高分子量多肽,通常是从胶原蛋白的水解中获得的不可逆变形式,与胶原蛋白相似,明胶结构中RGD残基的存在可促进细胞粘附,增殖和迁移,表现出较低的抗原性[26]。 明胶与藻酸盐混合可通过成分比例和温度的控制来提高可打印性(两种聚合物均具有热响应性),并有助于打印后的交联[27]。 藻酸盐 一种生物相容性良好的带负电荷的多糖,具有高剪切稀化和可快速胶凝后打印的性能[28]。 通过增加藻酸盐的粘度或使用如含Ca2+的化学交联剂提高可打印性[27];缺乏能使细胞粘附的RGD结构域,因此通常将天然蛋白(包括纤维蛋白,壳聚糖,胶原蛋白,HA和最常见的明胶)与藻酸盐混合[29]。 壳聚糖 一种在无脊椎动物和真菌的外骨骼中大量发现的多糖,止痛和止血的聚合物,也可用作抗菌、消炎药和伤口愈合剂[30]。 双挤压平台逐层沉积多糖壳聚糖和聚(γ-谷氨酸),壳聚糖的氨基和γ-谷氨酸的羧基之间形成的静电相互作用为打印结构提供了稳定性,使细胞具有良好的活力[31];非离子热敏交联,即将壳聚糖转化为羟丙基几丁质(HPCH),有利于细胞的存活和增殖[32]。 dECM 除去组织或器官的非细胞部分,为细胞的信息交流提供可调节的环境并支持细胞迁移。而且,ECM可以通过使用不同的方法获得,并重新用作组织再生的支架[33]。 使猪皮肤组织脱细胞形成了可打印的dECM生物墨水,可促进皮肤的稳定性,增强表皮组织,促进体内的新血管形成和再上皮形成以及伤口闭合[34];采用脱细胞工艺制备了猪皮粉,并将猪皮粉与具有良好可打印性的海藻酸盐混合,开发了新型生物墨水,打印的细胞显示出更高的代谢活性[35]。 GelMA GelMA可以方便地调节支架力学性能、表面硬度、孔隙率、降解性等,具有亲水性,可吸收渗出液,保持创面润洁,具有光敏特性和温敏特性[36]。 在光交联的明胶中培养永生化的人角质形成细胞制造了具有一定屏障功能的表皮[37];在不同结构的GelMA打印物培养了人脐静脉内皮细胞、小鼠成肌细胞和成纤维细胞,使细胞可以按照GelMA光固化形成的图案生长和增殖[38]。 表 2 用于皮肤修复的生物3D打印的主要细胞类型及应用
Table 2. The main cell types and applications of 3D bioprinting for skin repairing
细胞类型 简介 应用 角质形成细胞 表皮中的主要细胞类型。 Lee等人[45]利用胶原和角质形成细胞作为生物墨水,打印了伤口特异性组织工程皮肤产品;Kim等人[46]通过挤出打印和喷墨打印使用角质形成细胞直接打印了皮肤模型。 成纤维细胞 存在于皮肤的真皮层中,负责细胞外基质和非纤维成分的产生[47]。 Won等人[48]将基于皮肤脱细胞的细胞外基质(dECM)粉末和成纤维细胞作为生物墨水,发现细胞中的基因表达与皮肤形态生物学相似;Shi等人[49]通过挤压成型用藻酸钠/明胶复合材料和成纤维细胞制造了一种新型的真皮替代支架,与人体皮肤组织具有相似的理化特性。 内皮细胞 内皮细胞的存在可以增加血管化形成,有利于各种生长因子的分泌和信号交流。 Baltaza等人[50]在真皮层中合并了内皮细胞自组装的血管床,用于促进皮肤移植物的血管生成和灌注;Huyan等人[51]制造了由成纤维细胞、角质形成细胞和微血管内皮细胞组成的双层结构,具有显着的血管生成现象。 黑色素细胞 黑色素细胞,其分泌的黑色素可以作为调节皮肤颜色和抵御紫外线的应用。 Ng等人[52]应用3种不同类型的皮肤细胞(角质形成细胞,黑素细胞和成纤维细胞)制造了含色素的人体皮肤结构,并显示了与皮肤供体相似的浅色色素沉着;Min等人[53]将黑色素细胞和角质形成细胞打印在真皮层的顶部,并在真皮-表皮交界处观察到雀斑状色素沉着现象。 脂肪充间质干细胞 从脂肪组织中提取的具有多向分化潜能的干细胞。 显示出免疫调节炎症现象,促进新血管形成以及在再生过程中刺激增殖,还能够自我更新并分化为各种谱系,以替换受损组织[54-55]。 骨髓充间质干细胞 从骨髓穿刺物中分离出来的干细胞。 移植同种异体骨髓间充质干细胞后,几乎没有观察到排斥反应,可分化为角质形成细胞[56];骨髓充间质干细胞表达更高数量的胶原蛋白,可加速愈合过程,增加血管生成,以及直接分化成表达角质形成细胞特异性标记的上皮细胞来改善皮肤愈合[57-59]。 胎盘干细胞 从胎盘分离的干细胞。 胎盘获得的干细胞对供体没有风险,并且含有大量的再生细胞,具有免疫调节和免疫抑制特性[60]。 表 3 用于皮肤修复的主要生物3D打印技术及其用于皮肤修复的特点
Table 3. The main 3D bioprinting technology for skin repair and its characteristics
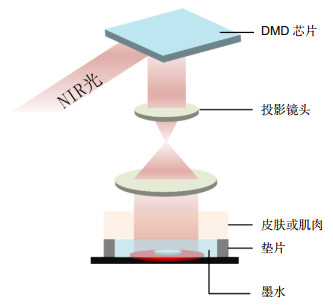
3D打印技术 所应用的主要光电技术 特点 喷墨打印 在无载体的情况下,精确沉积感光细胞,以预定的排列方式在细胞层上沉积了成熟的和分化的感光体[84];首先,通过调节施加到压电打印头的电压波形的参数来产生均匀的微滴。然后微滴被固化,从而形成微颗粒[85]。 打印精度高,可打印复杂的微米级结构;仅适用于低粘度生物材料墨水,交联困难,且细胞密度较低,否则会出现喷嘴堵塞。 光固化打印 数字微镜阵列设备(DMD)具有大约200万个微镜阵列,可以分别控制它们以控制在制造阶段投射到单体溶液的光学图案,并同时移动载物台,紫外光用于诱导光敏生物材料的光聚合[86-90]。 高分辨率,无喷嘴堵塞;材料需要与光引发剂混合,但是光引发剂会影响细胞活性。 激光辅助打印 利用激光能量将充满细胞的生物墨水,通过无接触无喷嘴的打印方式,将液滴推进到接收基质上[88, 91-92]。 无喷嘴,高细胞密度的精确沉积;高能量的激光束影响细胞活性。 挤出打印 利用激光扫描等技术实时监测挤出微丝的直径,便于调整工艺参数[49, 80, 93]。 生物材料墨水的可挤出粘度范围大,可混合的细胞密度高;挤出微丝的分辨率低,且挤出力会大大降低细胞存活率。 表 4 用于皮肤修复的主要生物3D打印技术及其用于皮肤修复的特点
Table 4. The main 3D bioprinting technology for skin repair and its characteristics

-
[1] Groeber F, Holeiter M, Hampel M, et al. Skin tissue engineering—In vivo and in vitro applications[J]. Adv Drug Deliv Rev, 2011, 63(4-5): 352-366. doi: 10.1016/j.addr.2011.01.005
[2] Vijayavenkataraman S, Lu W F, Fuh J Y H. 3D bioprinting of skin: a state-of-the-art review on modelling, materials, and processes[J]. Biofabrication, 2016, 8(3): 032001. doi: 10.1088/1758-5090/8/3/032001
[3] Peck M D. Epidemiology of burns throughout the World. Part Ⅱ: intentional burns in adults[J]. Burns, 2012, 38(5): 630-637. doi: 10.1016/j.burns.2011.12.028
[4] Beheshtizadeh N, Lotfibakhshaiesh N, Pazhouhnia Z, et al. A review of 3D bio-printing for bone and skin tissue engineering: a commercial approach[J]. J Mater Sci, 2019, 55(9): 3729-3749. doi: 10.1007/s10853-019-04259-0
[5] Derakhshanfar S, Mbeleck R, Xu K G, et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances[J]. Bioact Mater, 2018, 3(2): 144-156. doi: 10.1016/j.bioactmat.2017.11.008
[6] Tan S H, Ngo Z H, Leavesley D, et al. Recent advances in the design of three-dimensional and bioprinted scaffolds for full-thickness wound healing[J]. Tissue Eng Part B: Rev, 2021, doi: 10.1089/ten.TEB.2020.0339.
[7] Ozbolat I T. Bioprinting scale-up tissue and organ constructs for transplantation[J]. Trends Biotechnol, 2015, 33(7): 395-400. doi: 10.1016/j.tibtech.2015.04.005
[8] Chouhan D, Dey N, Bhardwaj N, et al. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances[J]. Biomaterials, 2019, 216: 119267. doi: 10.1016/j.biomaterials.2019.119267
[9] Clark R A F, Ghosh K, Tonnesen M G. Tissue engineering for cutaneous wounds[J]. J Invest Dermatol, 2007, 127(5): 1018-1029. doi: 10.1038/sj.jid.5700715
[10] Rodrigues M, Kosaric N, Bonham C A, et al. Wound healing: a cellular perspective[J]. Physiol Rev, 2019, 99(1): 665-706. doi: 10.1152/physrev.00067.2017
[11] Yannas I V, Tzeranis D, So P T. Surface biology of collagen scaffold explains blocking of wound contraction and regeneration of skin and peripheral nerves[J]. Biomed Mater, 2015, 11(1): 014106. doi: 10.1088/1748-6041/11/1/014106
[12] Bhardwaj N, Chouhan D, Mandal B B. Tissue engineered skin and wound healing: current strategies and future directions[J]. Curr Pharm Des, 2017, 23(24): 3455-3482. doi: 10.2174/1381612823666170526094606
[13] Liu T, Qiu C, Ben C, et al. One-step approach for full-thickness skin defect reconstruction in rats using minced split-thickness skin grafts with Pelnac overlay[J]. Burns Trauma, 2019, 7: 19. doi: 10.1186/s41038-019-0157-0
[14] Haflah N H M, Ng M H, Yunus M H M, et al. Massive traumatic skin defect successfully treated with autologous, bilayered, tissue-engineered MyDerm skin substitute: a case report[J]. JBJS Case Connect, 2018, 8(2): e38. doi: 10.2106/JBJS.CC.17.00250
[15] Kirchmajer D M, Gorkin III R, in het Panhuis M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing[J]. J Mater Chem B, 2015, 3(20): 4105-4117. doi: 10.1039/C5TB00393H
[16] Parak A, Pradeep P, du Toit L C, et al. Functionalizing bioinks for 3D bioprinting applications[J]. Drug Discov Today, 2019, 24(1): 198-205. doi: 10.1016/j.drudis.2018.09.012
[17] Agrawal P, Soni S, Mittal G, et al. Role of polymeric biomaterials as wound healing agents[J]. Int J Lower Extrem Wounds, 2014, 13(3): 180-190. doi: 10.1177/1534734614544523
[18] Gunatillake P A, Adhikari R. Biodegradable synthetic polymers for tissue engineering[J]. Eur Cells Mater, 2003, 5: 1-16. doi: 10.22203/eCM.v005a01
[19] Gugerell A, Pasteiner W, Nürnberger S, et al. Thrombin as important factor for cutaneous wound healing: comparison of fibrin biomatrices in vitro and in a rat excisional wound healing model[J]. Wound Repair Regen, 2014, 22(6): 740-748. doi: 10.1111/wrr.12234
[20] Currie L J, Martin R, Sharpe J R, et al. A comparison of keratinocyte cell sprays with and without fibrin glue[J]. Burns, 2003, 29(7): 677-685. doi: 10.1016/S0305-4179(03)00155-4
[21] Cubo N, Garcia M, del Cañizo J F, et al. 3D bioprinting of functional human skin: production and in vivo analysis[J]. Biofabrication, 2017, 9(1): 015006. doi: 10.1088/1758-5090/9/1/015006
[22] Donderwinkel I, van Hest J C M, Cameron N R. Bio-inks for 3D bioprinting: recent advances and future prospects[J]. Polym Chem, 2017, 8(31): 4451-4471. doi: 10.1039/C7PY00826K
[23] Thiele J, Ma Y J, Bruekers S M C, et al. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide[J]. Adv Mater, 2014, 26(1): 125-148. doi: 10.1002/adma.201302958
[24] Kim Y B, Lee H, Kim G H. Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a collagen/genipin-bioink and an optimal 3D printing process[J]. ACS Appl Mater Interfaces, 2016, 8(47): 32230-32240. doi: 10.1021/acsami.6b11669
[25] Lee J W, Choi Y J, Yong W J, et al. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering[J]. Biofabrication, 2016, 8(1): 015007. doi: 10.1088/1758-5090/8/1/015007
[26] Norouzi M, Boroujeni S M, Omidvarkordshouli N, et al. Advances in skin regeneration: application of electrospun scaffolds[J]. Adv Healthc Mater, 2015, 4(8): 1114-1133. doi: 10.1002/adhm.201500001
[27] Liu P C, Shen H Z, Zhi Y, et al. 3D bioprinting and in vitro study of bilayered membranous construct with human cells-laden alginate/gelatin composite hydrogels[J]. Colloids Surf B: Biointerfaces, 2019, 181: 1026-1034. doi: 10.1016/j.colsurfb.2019.06.069
[28] Del Amo C, Perez-Valle A, Perez-Zabala E, et al. Wound dressing selection is critical to enhance platelet-rich fibrin activities in wound care[J]. Int J Mol Sci, 2020, 21(2): 624. doi: 10.3390/ijms21020624
[29] Bociaga D, Bartniak M, Grabarczyk J, et al. Sodium alginate/gelatine hydrogels for direct bioprinting-the effect of composition selection and applied solvents on the bioink properties[J]. Materials, 2019, 12(17): 2669. doi: 10.3390/ma12172669
[30] Wang J, Shang P J, Shi W B, et al. Dissimilarity measure based on ordinal pattern for physiological signals[J]. Commun Nonlinear Sci Numer Simul, 2016, 37: 115-124. doi: 10.1016/j.cnsns.2016.01.011
[31] Pisani S, Dorati R, Scocozza F, et al. Preliminary investigation on a new natural based poly(gamma-glutamic acid)/Chitosan bioink[J]. J Biomed Mater Res Part B: Appl Biomater, 2020, 108(7): 2718-2732. doi: 10.1002/jbm.b.34602
[32] Li Y, Jiang X L, Li L, et al. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: scalable expansion and uniform aggregation[J]. Biofabrication, 2018, 10(4): 044101. doi: 10.1088/1758-5090/aacfc3
[33] Dzobo K, Motaung K S C M, Adesida A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: an updated review[J]. Int J Mol Sci, 2019, 20(18): 4628. doi: 10.3390/ijms20184628
[34] Kim B S, Kwon Y W, Kong J S, et al. 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering[J]. Biomaterials, 2018, 168: 38-53. doi: 10.1016/j.biomaterials.2018.03.040
[35] Lee S J, Lee J H, Park J, et al. Fabrication of 3D printing scaffold with porcine skin decellularized bio-ink for soft tissue engineering[J]. Materials, 2020, 13(16): 3522. doi: 10.3390/ma13163522
[36] 吴兴, 刘肇兴, 林欢欢, 等. 甲基丙烯酸酐明胶材料学特性及在皮肤组织工程应用与进展[J]. 中国组织工程研究, 2018, 22(2): 323-328. doi: 10.3969/j.issn.2095-4344.0025
Wu X, Liu Z X, Lin H H, et al. Properties of gelatin methacryloyl and its application in the skin tissue engineering[J]. Chin J Tissue Eng Res, 2018, 22(2): 323-328. doi: 10.3969/j.issn.2095-4344.0025
[37] Zhao X, Lang Q, Yildirimer L, et al. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering[J]. Adv Healthc Mater, 2016, 5(1): 108-118. doi: 10.1002/adhm.201500005
[38] Klotz B J, Gawlitta D, Rosenberg A J W P, et al. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair[J]. Trends Biotechnol, 2016, 34(5): 394-407. doi: 10.1016/j.tibtech.2016.01.002
[39] Bianco P, Robey P G. Stem cells in tissue engineering[J]. Nature, 2001, 414(6859): 118-121. doi: 10.1038/35102181
[40] Boyce S T, Medrano E E, Abdel-Malek Z, et al. Pigmentation and inhibition of wound contraction by cultured skin substitutes with adult melanocytes after transplantation to athymic mice[J]. J Invest Dermatol, 1993, 100(4): 360-365. doi: 10.1111/1523-1747.ep12471822
[41] Duncan C O, Shelton R M, Navsaria H, et al. In vitro transfer of keratinocytes: comparison of transfer from fibrin membrane and delivery by aerosol spray[J]. J Biomed Mater Res Part B: Appl Biomater, 2005, 73B(2): 221-228. doi: 10.1002/jbm.b.30198
[42] Sumorejo P, Listiawan M Y, Putri A I, et al. The role of stem cell metabolites derived from placenta for skin regeneration: an in vitro study[J]. Bali Med J, 2019, 8(1): 354-359. doi: 10.15562/bmj.v8i1.1387
[43] Mahmood R, Mehmood A, Choudhery M S, et al. Human neonatal stem cell-derived skin substitute improves healing of severe burn wounds in a rat model[J]. Cell Biol Int, 2019, 43(2): 147-157. doi: 10.1002/cbin.11072
[44] Metcalfe A D, Ferguson M W J. Skin stem and progenitor cells: using regeneration as a tissue-engineering strategy[J]. Cell Mol Life Sci, 2008, 65(1): 24-32. doi: 10.1007/s00018-007-7427-x
[45] Lee W, Debasitis J C, Lee V K, et al. Multi-layered culture of human skin fibroblasts and keratinocytes through three-dimensional freeform fabrication[J]. Biomaterials, 2009, 30(8): 1587-1595. doi: 10.1016/j.biomaterials.2008.12.009
[46] Kim B S, Lee J S, Gao G, et al. Direct 3D cell-printing of human skin with functional transwell system[J]. Biofabrication, 2017, 9(2): 025034. doi: 10.1088/1758-5090/aa71c8
[47] Nolte S V, Xu W, Rennekampff H O, et al. Diversity of fibroblasts-a review on implications for skin tissue engineering[J]. Cells Tissues Organs, 2008, 187(3): 165-176. doi: 10.1159/000111805
[48] Won J Y, Lee M H, Kim M J, et al. A potential dermal substitute using decellularized dermis extracellular matrix derived bio-ink[J]. Artif Cells, Nanomed, Biotechnol, 2019, 47(1): 644-649. doi: 10.1080/21691401.2019.1575842
[49] Shi L, Xiong L M, Hu Y Q, et al. Three-dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering[J]. Polym Eng Sci, 2018, 58(10): 1782-1790. doi: 10.1002/pen.24779
[50] Baltazar T, Merola J, Catarino C, et al. Three dimensional bioprinting of a vascularized and perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells[J]. Tissue Eng Part A, 2020, 26(5-6): 227-238. doi: 10.1089/ten.tea.2019.0201
[51] Huyan Y G, Lian Q, Zhao T Z, et al. Pilot study of the biological properties and vascularization of 3D printed bilayer skin grafts[J]. Int J Bioprint, 2020, 6(1): 246. doi: 10.18063/ijb.v6i1.246
[52] Ng W L, Qi J T Z, Yeong W Y, et al. Proof-of-concept: 3D bioprinting of pigmented human skin constructs[J]. Biofabrication, 2018, 10(2): 025005. doi: 10.1088/1758-5090/aa9e1e
[53] Min D, Lee W, Bae I H, et al. Bioprinting of biomimetic skin containing melanocytes[J]. Exp Dermatol, 2018, 27(5): 453-459. doi: 10.1111/exd.13376
[54] Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement[J]. Cytotherapy, 2006, 8(4): 315-317. doi: 10.1080/14653240600855905
[55] Kim H J, Park J S. Usage of human mesenchymal stem cells in cell-based therapy: advantages and disadvantages[J]. Dev Reprod, 2017, 21(1): 1-10. doi: 10.12717/DR.2017.21.1.001
[56] Kokubun K, Pankajakshan D, Kim M J, et al. Differentiation of porcine mesenchymal stem cells into epithelial cells as a potential therapeutic application to facilitate epithelial regeneration[J]. J Tissue Eng Regen Med, 2016, 10(2): E73-E83. doi: 10.1002/term.1758
[57] Strong A L, Neumeister M W, Levi B. Stem cells and tissue engineering: regeneration of the skin and its contents[J]. Clin Plastic Surg, 2017, 44(3): 635-650. doi: 10.1016/j.cps.2017.02.020
[58] Sasaki M, Abe R, Fujita Y, et al. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type[J]. J Immunol, 2008, 180(4): 2581-2587. doi: 10.4049/jimmunol.180.4.2581
[59] Wu Y J, Chen L W, Scott P G, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis[J]. Stem Cells, 2007, 25(10): 2648-2659. doi: 10.1634/stemcells.2007-0226
[60] Xiong J C, Ji B Y, Wang L J, et al. Human adipose-derived stem cells promote seawater-immersed wound healing by activating skin stem cells via the EGFR/MEK/ERK pathway[J]. Stem Cells Int, 2019, 2019: 7135974. doi: 10.1155/2019/7135974
[61] Visscher D O, Farré-Guasch E, Helder M N, et al. Advances in bioprinting technologies for craniofacial reconstruction[J]. Trends Biotechnol, 2016, 34(9): 700-710. doi: 10.1016/j.tibtech.2016.04.001
[62] Murphy S V, Atala A. 3D bioprinting of tissues and organs[J]. Nat Biotechnol, 2014, 32(8): 773-785. doi: 10.1038/nbt.2958
[63] Xu T, Jin J, Gregory C, et al. Inkjet printing of viable mammalian cells[J]. Biomaterials, 2005, 26(1): 93-99. doi: 10.1016/j.biomaterials.2004.04.011
[64] Park J A, Yoon S, Kwon J, et al. Freeform micropatterning of living cells into cell culture medium using direct inkjet printing[J]. Sci Rep, 2017, 7(1): 14610. doi: 10.1038/s41598-017-14726-w
[65] Yoon S, Park J A, Lee H R, et al. Inkjet-spray hybrid printing for 3D freeform fabrication of multilayered hydrogel structures[J]. Adv Healthc Mater, 2018, 7(14): 1800050. doi: 10.1002/adhm.201800050
[66] Xu C X, Zhang M, Huang Y, et al. Study of droplet formation process during drop-on-demand inkjetting of living cell-laden bioink[J]. Langmuir, 2014, 30(30): 9130-9138. doi: 10.1021/la501430x
[67] Negro A, Cherbuin T, Lutolf M P. 3D inkjet printing of complex, cell-laden hydrogel structures[J]. Sci Rep, 2018, 8(1): 17099. doi: 10.1038/s41598-018-35504-2
[68] Park J A, Lee H R, Park S Y, et al. Self-organization of fibroblast-laden 3D collagen microstructures from inkjet-printed cell patterns[J]. Adv Biosyst, 2020, 4(5): 1900280. doi: 10.1002/adbi.201900280
[69] Kim B S, Gao G, Kim J Y, et al. 3D cell printing of perfusable vascularized human skin equivalent composed of epidermis, dermis, and hypodermis for better structural recapitulation of native skin[J]. Adv Healthc Mater, 2019, 8(7): 1801019. doi: 10.1002/adhm.201801019
[70] Lim K S, Levato R, Costa P F, et al. Bio-resin for high resolution lithography-based biofabrication of complex cell-laden constructs[J]. Biofabrication, 2018, 10(3): 034101. doi: 10.1088/1758-5090/aac00c
[71] Zhang A P, Qu X, Soman P, et al. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography[J]. Adv Mater, 2012, 24(31): 4266-4270. doi: 10.1002/adma.201202024
[72] Ma X Y, Qu X, Zhu W, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting[J]. Proc Natl Acad Sci USA, 2016, 113(8): 2206-2211. doi: 10.1073/pnas.1524510113
[73] Zhu W, Qu X, Zhu J, et al. Direct 3D bioprinting of prevascularized tissue constructs with complex microarchitecture[J]. Biomaterials, 2017, 124: 106-115. doi: 10.1016/j.biomaterials.2017.01.042
[74] Zhou F F, Hong Y, Liang R J, et al. Rapid printing of bio-inspired 3D tissue constructs for skin regeneration[J]. Biomaterials, 2020, 258: 120287. doi: 10.1016/j.biomaterials.2020.120287
[75] Hribar K C, Soman P, Warner J, et al. Light-assisted direct-write of 3D functional biomaterials[J]. Lab Chip, 2014, 14(2): 268-275. doi: 10.1039/C3LC50634G
[76] Yan W C, Davoodi P, Vijayavenkataraman S, et al. 3D bioprinting of skin tissue: from pre-processing to final product evaluation[J]. Adv Drug Deliv Rev, 2018, 132: 270-295. doi: 10.1016/j.addr.2018.07.016
[77] Koch L, Kuhn S, Sorg H, et al. Laser printing of skin cells and human stem cells[J]. Tissue Eng Part C: Methods, 2010, 16(5): 847-854. doi: 10.1089/ten.tec.2009.0397
[78] Koch L, Deiwick A, Schlie S, et al. Skin tissue generation by laser cell printing[J]. Biotechnol Bioeng, 2012, 109(7): 1855-1863. doi: 10.1002/bit.24455
[79] Wang R, Wang Y H, Yao B, et al. Beyond 2D: 3D bioprinting for skin regeneration[J]. Int Wound J, 2019, 16(1): 134-138. doi: 10.1111/iwj.13003
[80] Mandrycky C, Wang Z J, Kim K, et al. 3D bioprinting for engineering complex tissues[J]. Biotechnol Adv, 2016, 34(4): 422-434. doi: 10.1016/j.biotechadv.2015.12.011
[81] Di Bella C, Duchi S, O'connell C D, et al. In situ handheld three-dimensional bioprinting for cartilage regeneration[J]. J Tissue Eng Regen Med, 2018, 12(3): 611-621. doi: 10.1002/term.2476
[82] Li X, Lian Q, Li D C, et al. Development of a robotic arm based hydrogel additive manufacturing system for in-situ printing[J]. Appl Sci, 2017, 7(1): 73. doi: 10.3390/app7010073
[83] Lee J, Kim K E, Bang S, et al. A desktop multi-material 3D bio-printing system with open-source hardware and software[J]. Int J Precis Eng Manuf, 2017, 18(4): 605-612. doi: 10.1007/s12541-017-0072-x
[84] Masaeli E, Forster V, Picaud S, et al. Tissue engineering of retina through high resolution 3-dimentional inkjet bioprinting[J]. Biofabrication, 2020, 12(2): 025006. doi: 10.1088/1758-5090/ab4a20
[85] Gao Q, He Y, Fu J Z, et al. Fabrication of shape controllable alginate microparticles based on drop-on-demand jetting[J]. J Sol-Gel Sci Technol, 2016, 77(3): 610-619. doi: 10.1007/s10971-015-3890-2
[86] Kumar H, Kim K. Stereolithography 3D Bioprinting[J]. Methods Mol Biol, 2020, 2140: 93-108.
[87] Warner J, Soman P, Zhu W, et al. Design and 3D printing of hydrogel scaffolds with fractal geometries[J]. ACS Biomater Sci Eng, 2016, 2(10): 1763-1770. doi: 10.1021/acsbiomaterials.6b00140
[88] Michael S, Sorg H, Peck C T, et al. Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice[J]. PLoS One, 2013, 8(3): e57741. doi: 10.1371/journal.pone.0057741
[89] Gauvin R, Chen Y C, Lee J W, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography[J]. Biomaterials, 2012, 33(15): 3824-3834. doi: 10.1016/j.biomaterials.2012.01.048
[90] Gou M L, Qu X, Zhu W, et al. Bio-inspired detoxification using 3D-printed hydrogel nanocomposites[J]. Nat Commun, 2014, 5: 3774. doi: 10.1038/ncomms4774
[91] Li J B, Han Y, Zhu J H. Influence equilibrium problem research based on common interests in mobile social network[C]//Proceedings of the 12th International Conference on Mobile Ad-Hoc and Sensor Networks, 2016: 271-278.
[92] Schiele N R, Corr D T, Huang Y, et al. Laser-based direct-write techniques for cell printing[J]. Biofabrication, 2010, 2(3): 032001. doi: 10.1088/1758-5082/2/3/032001
[93] Liu X, Michael S, Bharti K, et al. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies[J]. Biofabrication, 2020, 12(3): 035002. doi: 10.1088/1758-5090/ab76a1
[94] Lian Q, Li X, Li D C, et al. Path planning method based on discontinuous grid partition algorithm of point cloud for in situ printing[J]. Rapid Prototyping J, 2019, 25(3): 602-613. doi: 10.1108/RPJ-03-2018-0056
[95] Binder K W. In situ bioprintingi of the skin[D]. Winston-Salem: Wake Forest School, 2011: 1-443.
[96] Hakimi N, Cheng R, Leng L, et al. Handheld skin printer: in situ formation of planar biomaterials and tissues[J]. Lab Chip, 2018, 18(10): 1440-1451. doi: 10.1039/C7LC01236E
[97] Cheng R Y, Eylert G, Gariepy J M, et al. Handheld instrument for wound-conformal delivery of skin precursor sheets improves healing in full-thickness burns[J]. Biofabrication, 2020, 12(2): 025002. doi: 10.1088/1758-5090/ab6413
[98] Choi H S, Gibbs S L, Lee J H, et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging[J]. Nat Biotechnol, 2013, 31(2): 148-153. doi: 10.1038/nbt.2468
[99] Park G K, Kim S H, Kim K, et al. Dual-channel fluorescence imaging of hydrogel degradation and tissue regeneration in the brain[J]. Theranostics, 2019, 9(15): 4255-4264. doi: 10.7150/thno.35606
[100] Chen Y W, Zhang J M, Liu X, et al. Noninvasive in vivo 3D bioprinting[J]. Sci Adv, 2020, 6(23): eaba7406. doi: 10.1126/sciadv.aba7406
[101] Liu W J, Zhang Y S, Heinrich M A, et al. Rapid continuous multimaterial extrusion bioprinting[J]. Adv Mater, 2017, 29(3): 1604630. doi: 10.1002/adma.201604630
[102] Pi Q M, Maharjan S, Yan X, et al. Digitally tunable microfluidic bioprinting of multilayered cannular tissues[J]. Adv Mater, 2018, 30(43): 1706913. doi: 10.1002/adma.201706913
[103] Keriquel V, Guillemot F, Arnault I, et al. In vivo bioprinting for computer- and robotic-assisted medical intervention: preliminary study in mice[J]. Biofabrication, 2010, 2(1): 014101. doi: 10.1088/1758-5082/2/1/014101
[104] Albanna M, Binder K W, Murphy S V, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds[J]. Sci Rep, 2019, 9(1): 1856. doi: 10.1038/s41598-018-38366-w
[105] 陈凯云, 谢晓芹, 叶佩青. 航空压气机叶片型面在线激光测量系统设计[J]. 制造技术与机床, 2004(8): 53-56. doi: 10.3969/j.issn.1005-2402.2004.08.014
Chen K Y, Xie X Q, Ye P Q. Design on on-line laser measurement system for vane of aero-engine compressor[J]. Manuf Technol Mach Tool, 2004(8): 53-56. doi: 10.3969/j.issn.1005-2402.2004.08.014
[106] Li L, Yu F, Shi J P, et al. In situ repair of bone and cartilage defects using 3D scanning and 3D printing[J]. Sci Rep, 2017, 7(1): 9416. doi: 10.1038/s41598-017-10060-3
[107] Atala A, Kasper F K, Mikos A G. Engineering complex tissues[J]. Sci Transl Med, 2012, 4(160): 160rv12.
[108] Delrot P, Modestino M A, Gallaire F, et al. Inkjet printing of viscous monodisperse microdroplets by laser-induced flow focusing[J]. Phys Rev Appl, 2016, 6(2): 024003. doi: 10.1103/PhysRevApplied.6.024003
[109] Xue D, Wang Y C, Zhang J X, et al. Projection-based 3D printing of cell patterning scaffolds with multiscale channels[J]. ACS Appl Mater Interfaces, 2018, 10(23): 19428-19435. doi: 10.1021/acsami.8b03867
[110] Kattamis N T, Purnick P E, Weiss R, et al. Thick film laser induced forward transfer for deposition of thermally and mechanically sensitive materials[J]. Appl Phys Lett, 2007, 91(17): 171120. doi: 10.1063/1.2799877
[111] Guillotin B, Souquet A, Catros S, et al. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization[J]. Biomaterials, 2010, 31(28): 7250-7256. doi: 10.1016/j.biomaterials.2010.05.055
[112] Zhu Z J, Guo S Z, Hirdler T, et al. 3D printed functional and biological materials on moving freeform surfaces[J]. Adv Mater, 2018, 30(23): 1707495. doi: 10.1002/adma.201707495
[113] Suh Y J, Lim T H, Choi H S, et al. 3D printing and NIR fluorescence imaging techniques for the fabrication of implants[J]. Materials, 2020, 13(21): 4819. doi: 10.3390/ma13214819
-


 E-mail Alert
E-mail Alert RSS
RSS
 下载:
下载: